Abstract
Among vector-borne helminths, filarioids of the genus Dipetalonema (Spirurida: Onchocercidae) localize in several tissues and body cavities of several animal species, causing mild to moderate lesions. The pathological findings associated with Dipetalonema spp. infection in Neotropical monkeys from southern Brazil are herein described, along with a fatal case due to filarial polyserositis and entrapment of an intestinal segment. At necropsy, nematodes were observed in abdominal and thoracic cavities, or in the pericardium of 37 (31.3%) out of the 118 individuals examined (i.e., 35 Alouatta guariba clamitans and two Sapajus nigritus). In addition, at histology, 27.0% of positive animals presented microfilarie (inside blood vessels of lung, spleen, liver, and brain) and 8.1% presented adult nematodes in the heart, lung, and liver. In two cases, cross-sections of filarioids were associated with areas of epicardial thickening with intense fibrosis and pyogranulomatous inflammation in the brain, heart, liver, lungs, or spleen. The DNA fragment was amplify using the cox1 gene, sequenced and analyzed to identify the nematode species collected; presence of Wolbachia was assessed in the filarioids using the 16S rRNA gene. At BLAST analysis of the cox1 gene, 10 sequences showed 91.7% nucleotide identity with Dipetalonema gracile, and two with D. gracile (98.5%) and Dipetalonema graciliformis (98.3%). Phylogenetic analyses clustered sequences of the cox1 obtained in this study in two clades corresponding with the host species. Wolbachia sp. endosymbiont was detected in four samples. Data herein reported provide a description of pathological lesions associated with the infection by Dipetalonema spp., suggesting that they may cause disease in Neotropical monkeys. In addition, a better understanding of diversity and biology of Dipetalonema spp. in South America is needed to assess the impact they may cause in native non-human primates from Brazil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filarioids of the genus Dipetalonema (Spirurida: Onchocercidae) are parasites localized in the subcutaneous, body cavities, and other tissues of many animal species (Travi et al. 1985; Notarnicola et al. 2008), being vectored by arthropods, mainly of the genus Culicoides (Eberhard et al. 1979). These filarioids have been primarily described in wildlife animals, being most of them regarded as of minor or none pathogenicity (Travi et al. 1985; Karesh et al. 1998). In the Neotropical region, some Dipetalonema species (i.e., D. gracile, D. graciliformis, D. freitasi, D. caudispina, D. yatesi, and D. robini) have been collected in body cavities and several tissues (e.g., subcutaneous, lung, spleen) from monkeys of the genera Ateles, Cebus, Sapajus, Saimiri, Lagothrix, and Saguinus (Strait et al. 2012; Lefoulon et al. 2015; Vanderhoeven et al. 2017; Conga et al. 2018; Zárate-Rendón et al. 2022). Though primates infected with these filarioids do not display clinical signs, pathological lesions such as pleuritis, fibrinopurulent peritonitis, and fibrinous adhesion have been occasionally described (Travi et al. 1985; Strait et al. 2012; Baker 2019).
Neotropical monkeys are a diverse group of arboreal primates that inhabit tropical forests (Rosenberg and Hartwig 2013). In Brazil, monkeys of the genera Alouatta and Sapajus are distributed in different biomes, being often threatened due to anthropic pressures (e.g., dog attacks, hunting, road kills, and electric shock), as well as vector borne diseases, such as yellow fever (Moreno et al. 2015; Ehlers et al. 2022). Studies about the impact of filarioids on these animal species are scant and mainly limited to accidental macroscopic lesions (Bueno et al. 2017; Lopes et al. 2022). In this study, we describe pathological findings associated with Dipetalonema spp. infection in a large number of individuals sampled under the frame of a surveillance project on infectious diseases of Neotropical monkeys.
Methods
Sample collection
From April 2017 to October 2021, Alouatta guariba clamitans (n = 107) and Sapajus nigritus (n = 11) monkeys dead for different causes (e.g., dog attacks, road kills, electric shock, yellow fever, pneumonia, pleuropneumonia, hemorrhagic colitis, chronic renal failure, bacterial meningoencephalitis, and neoplasms) were collected and delivered to the Veterinary Pathology section of the Universidade Federal do Rio Grande do Sul for post-mortem examination. Animals came from zoological gardens, wildlife rescue centers, institutes of protection of wildlife, wildlife keepers, and veterinary clinics from Southern Brazil (see the “Acknowledgments” section).
Pathological and molecular analysis
Adult nematodes were collected from abdominal cavity, thoracic cavity, and/or pericardium, and subsequently frozen for further analysis. Nematodes collected at the necropsy were stored for further examination and fragments of organs (i.e., brain, diaphragm, heart, liver, lung, and spleen) were collected and fixed in 10% formalin solution. Afterwards, samples were embedded in paraffin, cut in 3-μm-thick slices and stained using the hematoxylin and eosin (HE). Genomic DNA from 12 filarioids (n=10 from A. guariba; n= 2 from S. nigritus) was extracted using the commercial kit Pure Link® Genomic DNA Mini Kit (Invitrogen TM, Carlsbad, CA, EUA) according to the manufacturer’s instructions. Samples were processed by conventional PCR (cPCR) assay using primers amplifying a portion (~689 bp) of partial mitochondrial cytochrome c oxidase subunit 1 (cox1) (Casiraghi et al. 2001). Thelazia callipaeda DNA was used as a positive control in PCR assays. In addition, samples were tested for Wolbachia spp. DNA through a cPCR targeting the 16S rRNA gene (Parola et al. 2003) and all PCR products were visualized by UV transilluminator following electrophoresis in 2% red-stained agarose gel.
Amplicons of the expected size were purified and sequenced in both directions using the Big Dye Terminator v.3.1 chemistry in a 3130 Genetic Analyzer (Applied Biosystems, California, USA) in an automated sequencer (ABI-PRISM 377). Consensus sequences were edited and compared with reference sequences available on GenBank database using the Basic Local Alignment Search Tool (BLAST). For each gene, sequences were selected for phylogenetic inferences based on BLAST results. The final dataset included 33 sequences, with Thelazia callipaeda selected as outgroup, and was aligned using the Fast Fourier transform algorithm in MAFFT (Katoh et al. 2019) using G-INS-I refinement method, and the ends were manually trimmed to unify their length. All parameters for phylogenetic analyses were treated as variables; therefore, GTR (the general time-reversible evolutionary model) was selected as the preferred evolutionary model to not a priori reduce the heuristic search. The shape parameter of the gamma distribution (G) and the proportion of invariable sites (I) was selected using jModelTest v 2.1.10 (Guindon and Gascuel 2003, Darriba et al. 2012), and the data were treated as partitioned, computing and applying the optimal substitution model for each position within codon individually. Phylogenetic analyses using maximum likelihood (ML) were computed employing RAxML v 8.1.12 (Stamatakis 2006, 2014). The best ML tree was selected from 100 iterations, and support for the branching pattern was validated through 103 pseudoreplicates. Phylogenetic analyses of Bayesian inference (BI) were carried out in MrBayes v 3.2 (Ronquist et al. 2012), and the resulting tree was constructed using the Metropolis-coupled Markov chain Monte Carlo algorithm. Four concurrent chains (one cold and three heated) ran for 106 generations, sampling trees every 100 generations. The first 30% of trees were discarded as a relative burn-in period after checking that the standard deviation split frequency fell below 0.01. Results were checked in Tracer v 1.7.1 (Rambaut et al. 2018) to assess convergence. Posterior probabilities were calculated as the frequency of samples recovering particular clades. The best evolutionary model was chosen under the Akaike information criterion using the iqTREE software (available at: http://iqtree.cibiv.univie.ac.at/). The phylogenetic analysis was performed by Bayesian analysis using CIPRES gateway (Ronquist and Huelsenbeck 2003). Protein translation for cox1 gene was performed with MEGA 11 software (Kumar et al. 2018) to check for whether the sequences contain incorrectly recognized nucleotides. Interspecific and intraspecific nucleotide divergence and the presence of haplotypes were evaluated according to criteria previously established by Ferri et al. (2009).
Results
Filarioid nematodes were detected in 37 (31.3%) individuals out of 118 primates necropsied, of which 35 were Southern Brown Howler Monkey (A. clamitans) and two Black-horned Capuchin (S. nigritus). Pathological gross lesions included polyserositis which it was characterized by proliferation of fibrosis (n = 31/37; 83.8%), and deposition of fibrinous material (n = 18/37; 48.6%) (Fig. 1a, b, c). In addition, serosa adhesion (n = 19/37; 51.4%) and hydrothorax (n = 9/37; 24.3%) were observed. Filiform nematodes (3.0–10.0 cm in length) were associated with polyserositis and were free and/or adhered to the surface of organs, in the abdominal cavity (Fig. 1d), thoracic cavity, and pericardium (Fig. 1e). In the intestine (n = 1/37; 2.7%), a focal area of fibrosis causes entrapment of the distal portion of the jejunum and the initial portion of the ileum (Fig. 1f). Characteristics and frequency of the lesions observed in the animals are reported in Table 1 and Supplementary file 1.
Gross lesions in Alouatta guariba clamitans and Sapajus nigritus monkeys infected by Dipetalonema spp. (a; case 2) Thoracic cavity with multifocal areas of fibrous adhesions in the visceral and parietal pleura associated with filarial nematodes (arrowhead) in an individual with polyserositis. (b; case 12) Thoracic cavity with proliferation of fibrous connective tissue in the visceral pleura causing adhesions in the lung. (c; case 7) Liver, marked proliferation of fibrous connective tissue in the form of fringes over the organ capsule. (d; case 13) Abdominal cavity, filarial nematodes in the mesentery. (e; case 13) Heart with epicardium presenting pale multifocal areas, and moderate adherence by fibrous and fibrinous serositis associated with filarial nematodes. (f; case 20) Small intestine with entrapment of intestinal segment by focal area of fibrosis with fibrous polyserositis caused by filarial nematodes
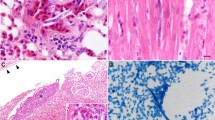
At the histology, microfilariae (n = 10/37; 27.0%) were detected inside blood vessels and capillaries of multiple organs, including the lung (Fig. 2a), spleen, liver, and brain, filled with multiple basophilic structures. Furthermore, cross-sections of adult nematodes (n = 3/37; 8.1%) covered by eosinophilic cuticle, with apparent coelomyarian muscles and presenting lateral cords, were observed (Fig. 2b), digestive and reproductive tracts. Nematodes were associated with areas of polyserositis in the heart (Fig. 2c), lung, and liver. In the lungs, pleural lesions were observed (Fig. 2d), being thickened due to fibrosis, fibrin deposition, and inflammatory pattern of different nature (i.e., lymphohistiocytic and eosinophilic, pyogranulomatous or neutrophilic). Epicardial thickening was characterized by intense fibrosis (Fig. 2e), fibrin deposition, associated with reactive mesothelial cells and predominantly lymphohistiocytic and eosinophilic inflammatory infiltrate. In two cases, cross-sections of adult forms of filarioids were observed in the epicardium.
Histological findings of Dipetalonema spp. infection in Alouatta guariba clamitans and Sapajus nigritus. (a; case 13) Lung, longitudinal section of 15 to 30 μm microfilariae in the lumen of capillary in alveolar septum. Hematoxylin and eosin (HE), × 400. (b; case 24) Cross section of adult female filarial nematode showing coelomyarian muscles (m), lateral cords (cl), intestine (i), and uterus (u) with developing microfilariae (arrowhead), attached to fibrous and fibrinous serositis associated with a moderate pyogranulomatous infiltrate. HE, × 400. (c; case 32) Heart, longitudinal section of an adult filarial nematode adhered to the epicardium (arrowhead) and associated with mild fibrous serositis (arrow). HE, × 40. (d; case 13) Lung, moderate thickening of visceral pleura due to proliferation of fibrous connective tissue and fibrin deposition, lymphohistiocytic and eosinophilic infiltrate (arrowhead) and focal mineralization (arrow). HE, × 200 (e; case 13) Heart, fibrous, and fibrinous serositis associated with inflammation (arrow), and filarial nematode cross-sections (arrowhead). HE, × 40. (f; case 32) Spleen, fibrous and fibrinous serositis associated with an intense pyogranulomatous infiltrate (arrow). HE, ×100
Liver presented thickened Glisson’s capsule, characterized by fibrosis covered by hyperplastic mesothelial cells, with fibrin deposition associated with a lymphohistiocytic and eosinophilic or pyogranulomatous inflammatory infiltrate. Similarly, longitudinal sections of microfilariae in the periportal region were associated with a mild inflammatory infiltrate, being predominantly lymphohistiocytic and eosinophilic.
Thickening of the spleen capsule was characterized by fibrosis, hyperplastic mesothelial cells, and fibrin deposition associated with a slight lymphohistiocytic and eosinophilic or pyogranulomatous inflammatory infiltrate (Fig. 2f).
At the BLAST analysis of the cox1 gene, nucleotide identity ranged from 91.7% with D. gracile (Accession number: KP760179) in 10 entries (i.e., detected in A. clamitans), to 98.5% and 98.3% with D. gracile (Accession number: KP760180) and D. graciliformis (Accession number: KP760182), in two sequences (i.e., detected in S. nigritus), respectively (Table 2). The cox1 sequences from the filarioids collected in the two primate species differed by the presence of seven silent mutations present in the two sequences from the specimens collected in S. nigritus. Based on this, the molecular results confirmed the species identification as Dipetalonema spp. Wolbachia sp. endosymbiont was detected in four samples (n= 2 A. guariba; n= 2 S. nigritus) presenting 99.4% nucleotide identity (100% query coverage) with Wolbachia sp. endosymbiont from D. gracile (Accession number: KU255234).
The final alignment for phylogenetic analyses spanned 610 unambiguously aligned nucleotide positions and the results of both statistical analyses (BI and ML) showed that nucleotide sequences obtained from this study clustered in two clades, ten in a single separate clade, and two in a clade with D. graciliformis (Fig. 3). All sequences obtained in this study were submitted in the GenBank database under the accession numbers: OQ508908 to OQ508919 (cox1), and OQ536153 to OQ536156 (16S rRNA).
Phylogenetic tree of filarioid species built from partial sequences of cox1 gene. Values at the nodes indicate posterior probabilities from BI and bootstrap values from ML analyses. Dashes indicate values below 0.70 and 50, respectively. Colored are sequences obtained from Dipetalonema sp. specimens within this study: violet = specimens from Alouatta guariba clamitans; red = specimens from Sapajus nigritus
Discussion
Data presented indicate that Dipetalonema spp. infections in Neotropical monkeys from Southern Brazil are associated with pathological lesions, such as those in the pericardium. In addition, a case of entrapment of an intestinal segment due to massive infection by these filarioids was also described, resulting in the death of a monkey (i.e., A. clamitans). Filarioids were mostly found in the abdominal cavity, as also reported in previous studies of Dipetalonema spp. in Neotropical primates (Notarnicola et al. 2008; Corrêa et al. 2016; Vanderhoeven et al. 2017; Lopes et al. 2022). Dipetalonema spp. have been morphologically described in A. guariba and Sapajus flavius from Rio Grande do Sul and Paraíba states, respectively (Bueno et al. 2017; Lopes et al. 2022), and D. gracile was molecularly characterized in Saguinus bicolor from Amazonas (Costa et al. 2023). Nonetheless, none of the above studies reported pathological findings. The presence of filarioids inside the pericardium seems to be the cause of major lesions in the epicardium, due to the intense fibrin deposition and fibrosis in eight primates. In addition, focal area of fibrosis in the mesentery of one individual may have been caused by the migration of the parasites, resulting in ischemic necrosis of the intestinal portion with consequent death of the monkey.
Pathological lesions associated with the presence of similar filarioid species have been occasionally described in humans. For example, chronic fibrous and fibrinous pericarditis were reported in people infected with Mansonella perstans adult worms (Simonsen et al. 2011; Mediannikov and Ranque 2018). In addition, polyserositis observed in animals from this study are similar to those described for M. perstans in humans, and by Litomosoides sigmodontis in rodents (Fercoq et al. 2019; Fercoq et al. 2020). Indeed, lesions caused by both nematode species have been associated with the development of the adult filarioids in the cavities (Jaquet 1980; Fercoq et al. 2020).
Infections by Dipetalonema spp. have been previously described in Neotropical primates causing mild peritonitis or chronic pleuritis, which are associated with areas of fibrous and occasionally, fibrinous adhesions (Chalifoux 1993; Strait et al. 2012). Similarly, other pathological findings (e.g., polyserositis, peritonitis, eosinophilic or lymphocytic infiltration) have been associated with filarial nematodes (i.e., Setaria tundra, Onchocerca flexuosa) in other hosts such as cervids from Finland, Japan, and Poland (Laaksonen et al. 2009; Kowal et al. 2013; Abd-Ellatieff et al. 2022). However, further description of the lesions was not performed to confirm whether the injuries were caused by the presence of the nematodes in the abdominal cavity (Laaksonen et al. 2007; Nikander et al. 2007).
The pathological lesions in the animals herein described could be an important health issue associated with Dipetalonema spp., since the filarioids infecting the primates from this study may cause clinical disease or even death depending on the infection intensity. These primates native to South America are considered threatened species (MMA 2014; Jerusalinsky et al. 2020), with populations affected by anthropic activities (e.g., dog attacks, hunting, road kills, and electric shock), and endemic diseases such as yellow fever (Chiarello and Galetti 1994; Bicca-Marques and de Freitas 2010). Therefore, confirming whether ubiquitous filarioids may represent a health problem for monkeys is of importance as a primary or concomitant cause of diseases in infected animals. In addition, though Wolbachia endosymbionts are implicated in inflammatory-mediated filarial infections (Taylor 2003; Manoj et al., 2021), its finding did not allow to draw any conclusions about their role in the occurrence of the lesions herein described.
Obtained sequences of the cox1 clearly separated the filarioids from A. clamitans and S. nigritus into separate clades. This fact, together with the low nucleotide identity with D. gracile (i.e., 91.7%), suggests that the specimens from A. clamitans may belong to a separate, presumably new, taxon within the genus Dipetalonema. Further studies should address in detail the morphology of the adult nematodes combined with further molecular analyses to confirm their taxonomic status and described this filarioid as a new species within the genus Dipetalonema.
Conclusion
Data herein reported provide detailed description of pathological lesions associated with the infection by filarioids of the genus Dipetalonema, proving that these nematodes are able to cause disease in free ranging Neotropical monkeys. Understanding the life cycle, vectors, and transmission ecology of these nematodes in local ecological context, together with more material from free-ranging primates, can answer arising questions about possible impact of filarioid parasites on populations of free-ranging primates, especially in a case of fragmented populations of threatened species.
Data availability
The authors declare that data supporting the findings of this study are available within the article.
References
Abd-Ellatieff HA, Bazh EK, Hussin SM, Yamamoto I, Yanai T, AbouRawash AA (2022) Onchocerca flexuosa. sp. (Nematoda: Filarioidea) in Japanese wild sika deer (Cervus nippon): pathological and molecular identification. J Parasit Dis 46(2):354–365. https://doi.org/10.1007/s12639-021-01453-3
Baker DG (2019) Parasitic Diseases. In: Marini R, Wachtman L, Tardif S, Mansfield K (eds) The common marmoset in captivity and biomedical, research. edn. Academic Press, USA
Bicca-Marques JC, de Freitas DS (2010) The role of monkeys, mosquitoes, and humans in the occurrence of a yellow fever outbreak in a fragmented landscape in South Brazil: protecting howler monkeys is a matter of public health. Trop Conserv Sci 3(1):78–89. https://doi.org/10.1177/194008291000300107
Bueno MG, Catão-Dias JL, de Oliveira LP, Arruda Vasconcellos S, Ferreira Neto JS, Gennari SM, Ferreira SM, Laurenti F, Umezawa MD, Kesper ES, Kirchgatter N, Guimarães KO, Pavanato L, Valença-Montenegro MM (2017) Infectious diseases in free-ranging blonde capuchins, Sapajus flavius, in Brazil. Int J Primatol 38(6):1017–1031. https://doi.org/10.1007/s10764-017-9994-5
Casiraghi M, Anderson TJC, Bandi C, Bazzocchi C, Genchi C (2001) A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitol 122:93–103. https://doi.org/10.1017/s0031182000007149
Chalifoux LV (1993) Filariasis, New World Primates. In: Jones TC, Mohr U, Hunt RD (eds) Nonhuman Primates I. Monographs on pathology of laboratory animals. Springer, Berlin, Heidelberg. Germany. https://doi.org/10.1007/978-3-642-84906-0_32
Chiarello A, Galetti M (1994) Conservation of the brown howler monkey in southeast Brazil. Oryx 28(1):37–42. https://doi.org/10.1017/S0030605300028271
Conga DF, Mayor P, Furtado AP, Giese EG, Santos JN dos (2018) Occurrence of Dipetalonema gracile in a wild population of woolly monkey Lagothrix poeppiigii in the northeastern Peruvian Amazon. Rev Bras Parasitol Vet 27 (2), 154–160. https://doi.org/10.1590/S1984-296120180014
Corrêa P, Bueno C, Soares R, Vieira FM, Muniz-Pereira LC (2016) Checklist of helminth parasites of wild primates from Brazil. Rev Mex Biodivers. 87(3):908–918. https://doi.org/10.1016/j.rmb.2016.03.008
Costa CHA, Crainey JL, Vicente ACP, Conga DF, Gordo M, Luz SLB, Dias CA, TRR da S, Ferreira CC, Nava AFD (2023) Ribosomal, mitochondrial and bacterial (Wolbachia) reference sequences for Dipetalonema gracile obtained from a wild pied tamarin (Saguinus bicolor) host in Manaus. Brazil. Acta Amazon. 53(2):130–140. https://doi.org/10.1590/1809-4392202201741
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, newheuristics and parallel computing. Nat Methods 9(8):772. https://doi.org/10.1038/nmeth.2109
Eberhard ML, Lowrie RC, Orihel TC (1979) Development of Dipetalonema Gracile and D. Caudispina to the Infective Stage in Culicoides Hollensis. J. Parasitol. 65(1):89–95. https://doi.org/10.2307/3280209
Ehlers LP, Slaviero M, Bianchi MV, de Mello LS, De Lorenzo C, Surita LE, Alievi MM, Driemeier D, Pavarini SP, Sonne L (2022) Causes of death in neotropical primates in Rio Grande do Sul State, Southern Brazil. J Med Primatol 51(2):85–92. https://doi.org/10.1111/jmp.12557
Fercoq F, Remion E, Frohberger SJ, Vallarino-Lhermitte N, Hoerauf A, Le Quesne J, Landmann F, Hübner MP, Carlin LM, Martin C (2019) IL-4 receptor dependent expansion of lung CD169+ macrophages in microfilaria-driven inflammation. PLoS Negl Trop Dis 30:13. https://doi.org/10.1371/journal.pntd.0007691
Fercoq F, Remion E, Vallarino-Lhermitte N, Alonso J, Raveendran L, Nixon C, Quesne JL, Carlin LM, Martin C (2020) Microfilaria-dependent thoracic pathology associated with eosinophilic and fibrotic polyps in filaria-infected rodents. Parasit Vectors 13(1):1–15
Ferri E, Barbuto M, Bain O, Galimberti A, Uni S, Guerrero R, Ferté H, Bandi C, Martin C, Casiraghi M (2009) Integrated taxonomy: traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda). Front Zool 6(1):1–12. https://doi.org/10.1186/1742-9994-6-1
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate largephylogenies by maximum likelihood. Syst Biol 52(5):696–704. https://doi.org/10.1080/10635150390235520
Jaquet C (1980) Litomosoides carinii (Travassos, 1919) Chandler, 1913 (Filarioidea) in Cotton Rats (Sigmodon hispidus, Say et Ord, 1825) and Jirds (Meriones unguiculatus, Milne-Edwards, 1867): comparison of the infection in relation to the immune response. Neuchâtel: Doctoral dissertation, University of Neuchâtel
Jerusalinsky L, Cortes-Ortíz L, de Melo FR, Miranda J, Alonso A, Buss G, Alves SL, Bicca-Marques J, Neves L, Ingberman B, Fries B, da Cunha R, Mittermeier RS, Talebi M (2020) .Alouatta guariba. The IUCN Red List of Threatened Species. e.T39916A17926390. (7)
Karesh WB, Wallace RB, Painter RLE, Rumiz D, Braselton WE, Dierenfeld ES, Puche H (1998) Immobilization and health assessment of free-ranging black spider monkeys (Ateles paniscus chamek). Am J Primatol 44(2):107–123. https://doi.org/10.1002/(SICI)1098-2345
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20(4):1160–1166. https://doi.org/10.1093/bib/bbx108
Kowal J, Kornaś S, Nosal P, Basiaga M, Lesiak M (2013) Setaria tundra in roe deer (Capreolus capreolus) new findings in Poland. Ann Parasitol 59(4):179–182
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Laaksonen S, Kuusela J, Nikander S, Nylund M, Oksanen A (2007) Outbreak of parasitic peritonitis in reindeer in Finland. Vet Rec 160(24):835–841. https://doi.org/10.1136/vr.160.24.835
Laaksonen S, Solismaa M, Orro T, KuuselaJ SS, Kortet R, Nokander S, Oksanen A, Sukura A (2009) Setaria tundra microfilariae in reindeer and other cervids in Finland. Parasitol Res 104:257–265
Lefoulon E, Bain O, Bourret J, Junker K, Guerrero R, Cañizales I, Kuzmin Y, Satoto TBT, Cardenas-Callirgos JM, Lima SS, Raccurt C, Mutafchiev Y, Gavotte L, Martin C (2015) Shaking the tree: multi-locus sequence typing usurps current onchocercid (filarial nematode) phylogeny. PLOS Negl Trop Dis 9(11). https://doi.org/10.1371/journal.pntd.0004233
Lopes S, Calegaro-Marques C, Klain V, Chaves ÓM, Bicca-Marques JC (2022) Necropsies disclose a low helminth parasite diversity in periurban howler monkeys. Am J Primatol 84(1). https://doi.org/10.1002/ajp.23346
Manoj RRS, Latrofa MS, Epis S, Otranto D (2021) Wolbachia: endosymbiont of onchocercid nematodes and their vectors. Parasit Vectors 14(1):245. https://doi.org/10.1186/s13071-021-04742-1
Mediannikov O, Ranque S (2018) Mansonellosis, the most neglected human filariasis. New Microbes New Infect 26:S19–S22. https://doi.org/10.1016/j.nmni.2018.08.016
MMA - Ministério do Meio Ambiente, Brasil (2014) Portaria no 443 de 17 de dezembro de 2014. Access in http://www.mma.gov.br/biodiversidade/especies-ameacadas-de-extincao/atualizacao-das-listas-especies-ameacadas. Accessed in march, 14 2023
Moreno ES, Agostini I, Holzmann I, Di Bitetti MS, Oklander LI, Kowalewski MM, Miller P (2015) Yellow fever impact on brown howler monkeys (Alouatta guariba clamitans) in Argentina. Metamodelling approach based on population viability analysis and epidemiological dynamics. Mem I Oswaldo Cruz 110:865–876. https://doi.org/10.1590/0074-02760150075
Nikander S, Laaksonen S, Saari S, Oksanen A (2007) The morphology of the filaroid nematode Setaria tundra, the cause of peritonitis in reindeer Rangifer tarandus. J Helminthol 81(01). https://doi.org/10.1017/s0022149x07214099
Notarnicola J, Pinto CM, Navone GT (2008) Host occurrence and geographical distribution of Dipetalonema spp. (Nematoda: Onchocercidae) in Neotropical monkeys and the first record of Dipetalonema gracile in Ecuador. Comp Parasitol 75(1):61–68. https://doi.org/10.1654/4284.1
Parola P, Cornet JP, Sanogo YO, Miller RS, Thien HV, Gonzalez JP, Raoult D, Telford SR III, Wongsrichanalai C (2003) Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J Clin Microbiol 41(4):1600–1608. https://doi.org/10.1128/JCM.41.4.1600-1608.2003
Rambaut lA, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67(5):901–904. https://doi.org/10.1093/sysbio/syy032
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542. https://doi.org/10.1093/sysbio/sys029
Rosenberger AL, Hartwig WC (2013) In: Wiley J, Sons L (eds) New World Monkeys in eLS, Chichester, UK
Simonsen PE, Onapa AW, Asio SM (2011) Mansonella perstans filariasis in Africa. Acta Trop:S109–S120. https://doi.org/10.1016/j.actatropica.2010.01.014
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogeneticanalyses with thousands of taxa and mixed models. Bioinformatics 22(21):2688–2690. https://doi.org/10.1093/bioinformatics/btl446
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post- analysis of large phylogenies. Bioinformatics 30(9):1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Strait KS, Else JG, Eberhard ML (2012) Parasitic diseases of nonhuman primates. In: Abee CR, Mansfield K, Tardif S, Morris T (eds) Nonhuman Primates in Biomedical Research Diseases. Academic Press, USA, San Diego
Taylor MJ (2003) Wolbachia in the inflammatory pathogenesis of human filariasis. Ann N Y Acad Sci 990(1):444–449
Travi BL, Eberhard ML, Lowrie RC (1985) Development of Dipetalonema gracile in the Squirrel Monkey (Saimiri sciureus), with Notes on Its Biology. J. Parasitol. 71(1):17–19. https://doi.org/10.2307/3281971
Vanderhoeven E, Notarnicola J, Agostini I (2017) Primer registro de Dipetalonema robini Petit, Bain & Roussilhon 1985 (Nematoda: Onchocercidae) parásito de Sapajus nigritus en el noreste de Argentina. Mastozool 24(2):483–488
Zárate-Rendón DA, Salazar-Espinoza MN, Catalano S, Sobotyk C, Mendoza AP, Rosenbaum M, Verocai G (2022) Molecular characterization of Dipetalonema yatesi from the black-faced spider monkey (Ateles chamek) with phylogenetic inference of relationships among Dipetalonema of Neotropical primates. IJP-PAW 17:152–157. https://doi.org/10.1016/j.ijppaw.2022.01.005
Acknowledgements
We are grateful to the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA-RS), Centro de Triagem de Animais Silvestres (CETAS-RS), Núcleo de Conservação e Reabilitação de Animais Silvestres (PRESERVAS–UFRGS), veterinarians and technicians from municipal and private zoo, local authorities, health surveillance centers, wildlife keepers, and veterinary clinics in the metropolitan region of Porto Alegre-RS.
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement. This study was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)–Financial code 001, Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), and Pro-Reitoria de Pesquisa of the Federal Universidade Federal do Rio Grande do Sul (Propesq/UFRGS). The coauthors LP, MABS, and DO were supported by the EU funding within the MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT). The coauthors JFS and LS are funded by Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico (CNPq Grant #312576/2021-8 #307277/2021-6, respectively).
Author information
Authors and Affiliations
Contributions
Luiza Presser Ehlers: conceptualization, investigation, methodology, writing—original draft preparation. Mônica Slaviero: investigation, methodology, writing—review and editing. Cíntia De Lorenzo: investigation, methodology, writing—review and editing. Renata Fagundes-Moreira: conceptualization, formal analysis, methodology, writing—original draft preparation. Viviane Kelin de Souza: formal analysis, methodology. Lívia Perles: formal analysis, methodology, writing—review and editing. Vinicius Baggio-Souza: formal analysis, methodology. Marcos Antonio Bezerra-Santos: formal analysis, investigation, methodology, supervision, writing—review and editing. David Modrý: formal analysis, supervision, writing—review and editing. Welden Panziera: investigation, supervision, writing—review and editing. David Driemeier: investigation, supervision, writing—review and editing. Saulo Petinatti Pavarini: investigation, supervision, writing—review and editing. João Fabio Soares: formal analysis, supervision, writing—review and editing. Domenico Otranto: conceptualization, formal analysis, methodology, supervision, writing—review and editing. Luciana Sonne: conceptualization, investigation, methodology, project administration, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
The procedures described here were conducted in accordance with the Research Committee of the Faculty of Veterinary of the Universidade Federal do Rio Grande do Sul, Brazil (n. 33660) and SISBIO License 76286. Furthermore, this research was conducted in accordance with the Principles of the American Society of Primatologists for the Ethical Treatment of Non-Human Primates.
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ehlers, L.P., Slaviero, M., De Lorenzo, C. et al. Pathological findings associated with Dipetalonema spp. (Spirurida, Onchocercidae) infection in two species of Neotropical monkeys from Brazil. Parasitol Res 122, 1973–1982 (2023). https://doi.org/10.1007/s00436-023-07895-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-023-07895-3