Abstract
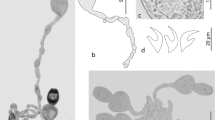
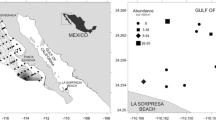
Larval didymozoids (Trematoda: Digenea) were discovered parasitizing the hemocoel of the heteropod Firoloida desmarestia (redia mean intensity = 13) and the chaetognaths Flaccisagitta enflata and Flaccisagitta hexaptera (metacercaria mean intensity = 1) during a 2014–2016 systematic study of parasites of zooplankton collected in the central and southern regions of the Gulf of California, Mexico. Didymozoid infection route during the early life cycle was inferred combining morphological (light microscopy) and molecular (mitochondrial cytochrome c oxidase subunit I gene, cox1) evidence. Didymozoid rediae parasitizing F. desmarestia were observed, just after field collection of the host, containing hundredths of completely developed cystophorous cercariae, releasing them though the birth pore at approximately one cercaria every 12 s. Cercariae lost their tails developing into a ‘young metacercaria’ in 1 d at 22 °C without need of an intermediate host. Molecular analysis of cox1 showed that rediae found in F. desmarestia belong to two distinct didymozoid species (Didymozoidae sp. 1 and sp. 2). Metacercariae parasitizing chaetognaths were morphologically identified as Didymozoidae type Monilicaecum and cox1 sequences showed that metacercariae of chaetognaths matched with these two Didymozoidae sp. 1, and sp. 2 species found parasitizing F. desmarestia, plus a third distinct Didymozoidae sp. 3. These are the first DNA sequences of cox1 gene from didymozoid larvae for any zooplankton taxonomic group in the world. We concluded that F. desmarestia is the first intermediate host of rediae and cercariae, and the chaetognaths are the second intermediate hosts where non-encysted metacercariae were found. The definitive host is still unknown because cox1 sequences of present study did not genetically match with any available cox1 sequence of adult didymozoid. Our results demonstrate a potential overlap in the distribution of two carnivorous zooplankton taxonomic groups that are intermediate hosts of didymozoids in the pelagic habitat. The didymozoid specimens were not identified to species level because any of the cox1 sequences generated here matched with the sequences of adult didymozoids currently available in GenBank and Bold System databases. This study provides baseline information for the future morphological and molecular understanding of the Didymozoidae larvae that has been previously based on the recognition of the 12 known morphotypes.



Similar content being viewed by others
Data availability
Data of the present manuscript are available upon request to the first author.
References
Abe N, Okamoto M, Maehara T (2014) Molecular characterization of muscle-parasitizing didymozoids in marine fishes. Acta Parasitol 59:354–358. https://doi.org/10.2478/s11686-014-0234-2
Al-Bassel DA, Ouhida ASB (2006) Light and TEM studies on gills of Tuna infecting with Allodidymozoon pharyngi (Didymozoidae trematodes) from Lybia. J Egiptian Germ Soc Zool 52A:117–129. https://www.fayoum.edu.eg/Scien/Zoology/pdf/DrDayhoum31.pdf
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Alvariño A (1963) Quetognatos epiplanctónicos del Mar de Cortés. Rev Soc Mex Hist Nat 24:97–203
Anderson GR, Barker SC (1993) Species differentiation in the didymozoidae (Digenea): restriction fragment length differences in internal transcribed spacer and 5.8S ribosomal DNA. Int J Parasitol 23:133–136. https://doi.org/10.1016/0020-7519(93)90106-9
Anderson GR, Barker SC (1998) Inference of phylogeny and taxonomy within the Didymozoidae (Digenea) from the second internal transcribed spacer (ITS2) of ribosomal DNA. Syst Parasitol 41:87–94. https://doi.org/10.1023/A:1006024128098
Anderson GR, Cribb TH (1994) Five new didymozoid trematodes (Platyhelminthes, Digenea) from Australian platycephalid fishes. Zool Scripta 23(2):83–93. https://doi.org/10.1111/j.1463-6409.1994.tb00377.x
Angulo-Campillo O, Aceves-Medina G, Avendaño-Ibarra R (2011) Holoplanktonic mollusks (Mollusca: Gastropoda) from the Gulf of California, México. Check List 7:337–342. https://doi.org/10.15560/7.3.337
Bárcenas de los Santos NY, Morales-Serna FN, Medina-Guerrero RM, Hernández-Covarrubias V, Oceguera-Figueroa A, García-Prieto L (2021) Helminth fauna of Scomberomorus sierra (Actinopterygii: Scombridae) in southeastern Gulf of California. Mexico Helminthologia 58(4):403–407. https://doi.org/10.2478/helm-2021-0035
Bieri R (1991) Systematics of the Chaetognatha. In: Bone Q, Kapp H, Pierrot-Bults AC (eds) The biology of Chaetognaths. Oxford Science Publications, USA, pp 122–136
Blair D, Bray RA, Barker SC (1998) Molecules and morphology in phylogenetic studies of the Hemiuroidea (Digenea: Trematoda: Platyhelminthes). Mol Phylogen Evol 9:15–25. https://doi.org/10.1006/mpev.1997.043.7
Bowles J, Blair D, Mcmanus DP (1992) Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol 54:165–173. https://doi.org/10.1016/0166-6851(92)90109-W
Cable RM (1956) Marine cercariae of Puerto Rico. Sci Surv Porto Rico Virgin Islands 16:491–577
Chero J, Cruces C, Iannacone K, Sáez G, Sanchez L, Minaya D, Alvariño L, Mendoza-Vidaurre C, Luque JJ (2015) First record of Unitubulotestis pelamydis (Trematoda: Didymozoidae) and Sphyriocephalus tergestinus (Cestoda: Sphyriocephalidae) in eastern Pacific bonito, Sarda chiliensis (Perciformes: Scombridae) in Peru. Neotrop Helminthol 9:313–323
Cort WW, Nichols EB (1920) A new cystophorous cercaria from California. J Parasitol 7:8–15. https://doi.org/10.2307/3271150
Cribb TH, Pichelin S, Dufour V, Bray RA, Chauvet C, Faliex E, Galzin R, Lo CM, Lo-Yat A, Morand S, Rigby MC, Sasal P (2000) Parasites of recruiting coral reef fish larvae in New Caledonia. Int J Parasitol 30:1445–1451. https://doi.org/10.1016/S0020-7519(00)00121-1
Felizardo NN, Justo MC, Knoff M, Fonseca MC, Pinto RM, Gomes DC (2011) Juvenile didymozoids of the types, Torticaecum and Neotorticaecum (Didymozoidae: Digenea), from new marine fish hosts (Pisces: Teleostei) in the neotropical region of Brazil. J Helminthol 85:270–275. https://doi.org/10.1017/S0022149X10000507
Gómez del Prado-Rosas MC, Álvarez-Cadena JN, Segura-Puertas L, Lamothe-Argumedo R (1999) First record of Torticaecum sp. (Trematoda: Didymozoidae) in the chaetognath Serratosagitta serratodentata (Krohn, 1853) from Caribbean waters. J Plankton Res 21:1005–1008. https://doi.org/10.1093/plankt/21.5.1005
Gómez del Prado-Rosas MC, Álvarez-Cadena JN, Segura-Puertas L, Lamothe-Argumedo R (2007) Didymozoid Monilicaecum type trematodes in chaetognaths from the Mexican Caribbean Sea. Rev Mex Biodiv 78:483–487
Ivanova NV, Dewaard JR, Hebert PDN (2006) An inexpensive, automation friendly protocol for recovering high-quality DNA. Mol Ecol Notes 6:998–1002. https://doi.org/10.1111/j.1471-8286.2006.01428.x
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequences data. Bioinformatics 28:1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Køie M (1995) The life-cycle and biology of Hemiurus communis Odhner, 1905 (Digenea: Hemiuridae). Parasite 2:195–202. https://doi.org/10.1051/parasite/199502s2195
Køie M, Lester RJG (1985) Larval Didymozoids (Trematoda) in fishes from Moreton Bay, Australia. Proc Helminthol Soc Wash 52:196–203
Køie M, Karlsbakk E, Nylund A (2002) A cystophorous cercaria and metacercaria in Analis entails (L.) (Mollusca, Scaphopoda) in Norwegian waters, the larval stage of Lecithophyllum botryophorum (Olsson, 1868) (Digenea, Lecithasteridae). Sarsia 87:302–311. https://doi.org/10.1080/00364820260400807
Kurochkin YV, Nikolaeva VM (1978) A classification of didymozoid metacercariae. In: First All Union Congress of Parasito-Coenologists. Part 3. Naukova Dumka, Kiev, pp. 82–84 (In Russian)
Lester RJ, Newman LJ (1986) First rediae and cercariae to be described from Heteropods. J Parasitol 72:95–197. https://doi.org/10.2307/3281823
Louvard C, Cutmore SC, Yong RQY, Dang C, Cribb TH (2022) First elucidation of a didymozoid life cycle: Saccularina magnacetabula n. gen. n. sp. infecting an arcid bivalve. Int J Parasitol 52(7):407–425. https://doi.org/10.1016/j.ijpara.2021.11.012
Lozano-Cobo H, Gómez del Prado-Rosas MC, Sánchez-Velasco L, Gómez-Gutiérrez J (2017) Seasonal variation in chaetognath and parasite species assemblages along the northeastern coast of the Yucatan Peninsula. Dis Aquat Org 124:55–75. https://doi.org/10.3354/dao03106
Lozano-Cobo H, Gómez-Gutiérrez J, Franco-Gordo C, Gómez del Prado-Rosas MC, Plascencia-Palomera V, Ambriz-Arreola I (2018) Changes in parasite-chaetognath species assemblages in the Mexican Central Pacific before and during El Niño 1997–1998. Dis Aquat Org 129:215–238. https://doi.org/10.3354/dao03245
Madhavi R (1968) A Didymozoid metacercariae from the copepod, Paracalanus aculeatus Giesbrecht, from Bay of Bengal. J Parasitol 54:629. https://doi.org/10.2307/3277098
Melo FTV, Silva JP, Goncalves EC, Furtado AP, Giese EG, Santos CP, Santos JN (2013) Taxonomic status and redescription of the genus Brasicystis Thatcher, 1979 (Digenea: Didymozoidae). Parasitol Int 62:208–214. https://doi.org/10.1016/j.parint.2013.01.001
Miura O, Kuris AM, Torchin ME, Hechinger RF, Dunhman EJ, Chiba S (2005) Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse). Int J Parasitol 35:793–801. https://doi.org/10.1016/j.ijpara.2005.02.014
Mladineo I (2006) Histopathology of five species of Didymocystis spp. (Digenea, Didymozoidae) in cage-reared Atlantic bluefin tuna (Thunnus thynnus thynnus). Vet Res Commun 30:475–484. https://doi.org/10.1007/s11259-006-3207-6
Mladineo I, Bott NJ, Nowak BK, Block BA (2010) Multilocus phylogenetic analyses reveal that habitat selection drives the speciation of Didymozoidae (Digenea) parasitizing Pacific and Atlantic bluefin tunas. Parasitology 137:1013–1015. https://doi.org/10.1017/S0031182009991703
Morales-Ávila JR, Saldierna-Martínez RJ, Moreno-Alcántara M, Violante-González J (2018) New insights on the role of the holoplanktonic mollusk Firoloida desmarestia (Gastropoda: Pterotracheidae) as host for digenetic trematodes. Parasitol Res 117:2149–2158. https://doi.org/10.1007/s00436-018-5902-y
Newman LJ (1990) Holoplanktonic molluscs (Gastropoda; Thecosomata, Gymnosomata and Heteropoda) from the waters of Australia and Papua New Guinea: their taxonomy, distribution and biology. Dissertation, University of Queensland, Australia p. 191
Nikolaeva VM (1965) On the developmental cycle of trematodes belonging to the family Didymozoidae. Zool Zhurnal 44:1317–1327
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ (2003) Phylogeny and classification of the Digenea. Int J Parasitol 33:733–755. https://doi.org/10.1016/S0020-7519(03)00049-3
Øresland V, Bray RA (2005) Parasites and headless chaetognaths in the Indian Ocean. Mar Biol 147:725–734. https://doi.org/10.1007/s00227-005-1618-5
Overstreet RM, Hochberg FG (1975) Digenetic trematodes in cephalopods. J Mar Biol Assoc UK 55:893–910. https://doi.org/10.1017/S0025315400017781
Pascual S, Abollo E, Azevedo C (2006) Host-parasite interaction of a muscle-infecting didymozoid in the Atlantic mackerel Scomber scombrus L. ICES J Mar Sci 63:169–175. https://doi.org/10.1016/j.icesjms.2005.08.010
Pérez-Ponce de León G, Hernández-Mena DIG (2019) Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the `next generation´ Tree of life. J Helminthol 93:260–276. https://doi.org/10.1017/S0022149X19000191
Pozdnyakov SE, Gibson DI (2008) Family Didymozoidae Monticelli, 1888. In: Bray RA, Gibson DI, Jones A (eds) Keys to the trematoda, Vol 3. CAB Int Nat Hist Mus. London, pp 631–734. https://doi.org/10.1079/9780851995885.0631
Reimer LW, Gerger C, Hener B, Lainka H, Rosenthal I, Scharnweber L (1971) On the distribution of larval helminths in planktonic animals of the North Sea. Parazitologiya (leningrand) 5:542–550
Reimer LW, Hnatiuk S, Rochner J (1975) Metacercarien in planktieren des mittleren Atlantik. Wissens chafliche Zeitschrift der Padagogischen Hochshule “Liselotte Herrmann”. Gustrow Aus der Methematisch – Naturwissenschaftlichen Fakultat 2:239–258
Rodríguez-Ibarra E, Monks S, Pulido-Flores G (2011) Metacecarias del tipo Paramonilicaecum (Digenea, Didymozoidae), parásitos accidentales de elasmobranquios (Elasmobranchii) del golfo de México e identificación de metacercarias (Didymozoidae) de la Colección Nacional de Helmintos. Rev Mex Biodiv 82(2):705–708
Schrandt MN, Andres MJ, Powers SP, Overstreet RM (2016) Novel infection site and ecology of cryptic Didymocystis sp. (Trematoda) in the fish Scomberomorus maculatus. J Parasitol 102:297–305. https://doi.org/10.1645/15-772
Seapy RC, Lalli CM, Wells FE (2003) Heteropoda from western Australian waters. In: Wells FE, Walker DI, Jones DS (eds) The marine flora and fauna of dampier, Western Australia. Western Australian Museum, Perth, pp 513–546
Shameem U, Rao NN, Madhavi R (1990) Cercaria chilkaensis No. 1, a new cystophorous cercaria from the snail Stenothyra blanfordiana from Chilka lake, India. J Nat Hist 24(1):261–270. https://doi.org/10.1080/00222939000770171
Shimazu T (1978) Some helminth parasites of the Chaetognatha from Suruga Bay, Central Japan. Bull Natl Sci Mus Tokyo Ser A Zool 4:105–116
Smith P, Richardson S (1977) Standard techniques for pelagic fish egg and larva surveys. FAO Fish Tech Pap 175:1–107
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9):1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (* and other methods). Sunderland, MA: Sinauer Associates, Version 4:b10
Tolonen A, Karlsbakk E (2003) Parasites of herring (Clupea harengus L.) larvae from a local Norwegian fjord stock. Sarsia 88:154–157. https://doi.org/10.1080/00364820310001327
Yamaguti S (1971) Synopsis of digenetic Trematodes of vertebrates. Keigaku Publishing, Tokyo, Japan, Parts I, II
Yip SY (1984) Parasites of Pleurobrachia pileus Muller, 1776 (Ctenophora), from Galway Bay, western Ireland. J Plankton Res 6:107–120. https://doi.org/10.1093/plankt/6.1.107
Acknowledgements
We thank Captain Pascual Barajas and the crew of the R/V El Puma and graduate students and researchers of the Fisheries Ecology Laboratory of the Instituto de Ciencias del Mar y Limnología (UNAM, Mexico City) and CICIMAR-IPN for their assistance with the zooplankton sampling. We deeply thank María del Carmen Gómez del Prado-Rosas for the facilities to do technical parasitological work at the Parasitology Laboratory (UABCS, La Paz, BCS, Mexico) and Clarisse Louvard (University of Queensland) by sharing valuable information about her publication Louvard et al. (2022). We deeply thank Dra. Patricia Cortés-Calva and M.Sc. Griselda F. Gallegos-Simental for the technical work extracting, amplifying, and sequencing the trematode specimens at the Nodo del Código de Barras de la Vida, MEXBOL at CIBNOR, La Paz, BCS, Mexico.
Funding
The Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas (SIP 2014–2022), Consejo Nacional de Ciencia y Tecnología-Ciencia Básica (2012–178615–C01, 2016–284201-C01) (projects administrator J.G.-G.), and Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México (PAPIIT, UNAM grant IN20066610–3 and CONACyT Ciencia Básica 2010–152850–C01, A1-S-21459) (projects administrator C.J.-R.) provided research funds to do the present study. We thank the project MEXBOL Barcode of Life to sequence zooplankton and their parasites in Los Cabos region; Project Code “ZPCR” for financial support the molecular analysis of parasites used in the present study Código de Barras de la Vida MEXBOL (project administrator J.G.-G.), and CONACyT 2018–295569, (project administrator Patricia Cortés-Calva). CONACyT Red Temática Código de barras de la vida de México (2011) also provided funds to the present study. H.L.-C. was supported by a PhD CONACyT (A14618), PIFI-IPN (SIP20110012) and BEIFI-IPN (SIP20140497, 20150113, 20160495, 20171275) doctoral grants. All the authors are SNI fellows, and J.G.-G. is also a COFAA-IPN and EDI-IPN fellow.
Author information
Authors and Affiliations
Contributions
Horacio Lozano Cobo, original idea, sampling and specimen transportation, morphological identification of parasites and hosts, preparation of manuscript and edition of figures, text and videos; Claudia Alicia Silva-Segundo, molecular analysis and text edition; Alejandro Oceguera-Figueroa, molecular analysis and text edition; Carlos Jorge Robinson, sampling and specimens transportation, text edition and project administrator; Jaime Gómez-Gutiérrez, original idea, sampling and specimen transportation, preparation of manuscript and text and figure and video edition, and project administrator.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The zooplankton sampling and specimen analysis protocols that were developed in the present study fully complied with the ethical principles of animal experimentation prepared by regulations of the Mexican government.
Consent to participate
The authors declare that they provide consent to participate in the present manuscript.
Consent for publication
The authors declare that they provide consent for publication of the present manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 129392 KB)
Supplementary file3 (MP4 128317 KB)
Supplementary file4 (MP4 86206 KB)
Supplementary file5 (MP4 147943 KB)
Rights and permissions
About this article
Cite this article
Lozano-Cobo, H., Oceguera-Figueroa, A., Silva-Segundo, C.A. et al. Finding a needle in a haystack: larval stages of Didymozoidae (Trematoda: Digenea) parasitizing marine zooplankton. Parasitol Res 121, 2661–2672 (2022). https://doi.org/10.1007/s00436-022-07593-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07593-6