Abstract
Toxoplasma gondii is a worldwide prevalent parasite. The infection has been linked to variable inflammatory effects including neuroinflammation. Biochanin A (BCA) is an isoflavone, known for its anti-inflammatory and anti-oxidative properties. In this study, we examined the effect of BCA on the brain and liver inflammatory lesions in a murine model with chronic toxoplasmosis. Mice were divided in to six groups: non-infected control, non-infected BCA-treated, and four infected groups with Toxoplasma gondii Me49-type II cystogenic strain: infected control, BCA (50 mg/kg/day)-treated, combined BCA/cotrimoxazole-treated and cotrimoxazole (370 mg/kg/day) alone-treated. Gene expression of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and inducible nitric oxide synthase (iNOS) was evaluated by quantitative real-time PCR in the brain and liver tissues. In the infected control group, an upregulation of TNF-α and IL-1β mRNA expression levels was found. However, a downregulation of iNOS expression was detected in the brain of infected control mice. In both BCA- and combined-treated groups, the brain and liver tissues showed significantly reduced inflammatory lesions compared to the infected control mice with inhibited TNF-α and IL-1β mRNA levels. The iNOS expression levels in the brain tissues of BCA group were significantly higher than the levels of the infected control group. BCA alone or combined significantly reduced T. gondii cyst count in the brain tissues. In conclusion, the anti-inflammatory activity of BCA was demonstrated in the brain tissues of mice with chronic toxoplasmosis with decreased TNF-α and IL-1β expression levels and increased iNOS expression levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii (T. gondii) is an opportunistic parasite, causing little or no apparent disease manifestations in immunocompetent individuals. However, during congenital infection or immunodeficiency, toxoplasmosis could lead to serious outcomes (Denkers 1999). The worldwide prevalence of toxoplasmosis was estimated to range between 10 and 80% (Pappas et al. 2009).
The intracellular parasites such as T. gondii, Leishmania sp. and Trypanosoma cruzi were recognized to induce activation of the host cells’ mitogen-activated protein kinase (MAPK) signaling pathways and induction of proinflammatory cytokines in macrophages (Valère et al. 2003; Kim et al. 2005; Nogueira et al. 2015). MAPKs are a family of serine/threonine kinases that include stress-activated protein kinase/JNK, ERKs, and p38, which are essential regulators of immunity and associated with numerous cell functions such as cytokine expression, cell proliferation, and apoptosis (Lu et al. 2017).
Th1 immune response against T. gondii infection is mediated by proinflammatory cytokines including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-1beta (IL-1β) (Suzuki and Remington 1988), inducing the infiltration of immune cells as CD8 + T cells into the brain (Schlüter et al. 2001). Inducible nitric oxide synthase (iNOS) with reactive nitrogen intermediates production has demonstrated a pivotal role in controlling chronic toxoplasmosis (Scharton-Kersten et al. 1997; Silva et al. 2009).
The expression of inflammatory cytokines is induced to keep the dormancy of T. gondii, although they could cause a subsequent neuromodulation and host behavior changes (Dunn 2006; Liesenfeld et al. 2011). Furthermore, inflammatory responses could affect uninfected neurons and disrupt the synaptic transmission (McCusker and Kelley 2013). Upregulation of pro-inflammatory biomarkers was implicated in the neuropsychiatric disorders (Lang et al. 2018). According to Mahmoudvand et al. (2016), the mRNA levels of TNF-α, IL-1β, and IL-6 were significantly increased in Toxoplasma-infected mice compared to uninfected mice. Those cytokines were described as contributing factors for hyperalgesia and neuroinflammation in mice brain tissues.
Biochanin A (BCA), 5,7-dihydroxy-4′-methoxyisoflavone, the methylated precursor of genistein, is found mainly in alfalfa and red clover (Lam et al. 2004). The flavonoids’ anti-inflammatory effects had been documented (Kole et al. 2011; Saviranta et al. 2011).
BCA inhibits the activation of MAPK pathway, leading to nuclear factor-κB (NF-κB)-driven inhibition of gene transcription and decreased expression of TNF-α, IL-1β, IL-6, iNOS, COX-2, and MMP-9 (Lam et al. 2004; Kole et al. 2011; Breikaa et al. 2013; Bhardwaj et al. 2014; Wu et al. 2018).
In this study, we investigated the effect of BCA, as a promising natural compound, on the inflammatory process of experimental chronic toxoplasmosis in the brain and the liver tissues. In addition, we aimed to assess the impact of BCA treatment on the parasitic load of the brain.
Materials and methods
Parasite
Mice were infected with Toxoplasma gondii Me49-type II cystogenic strain using a 22-gauge blunt feeding needle by intragastric inoculation of toxoplasmic cyst-containing brain homogenate, adjusted to 10 tissue cysts.
Experimental design, animals, drugs, and dosing
Female Swiss Webster mice were purchased from the Medical Experimental Research Center, Mansoura University (age, 6 to 8 weeks; weight, 18 to 20 g), maintained at 20–23 °C in an air-conditioned laboratory and provided ad libitum with standard commercial pelleted diet and water. Mice were divided into six groups (10 mice each). Group (G) IA: uninfected control mice. G IB: uninfected BCA-control. G II: infected control, vehicle-treated (0.5 mL carboxy-methylcellulose). G III: infected treated with biochanin A (BCA), purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). BCA (50 mg/kg body weight/day) was suspended in 0.5% carboxymethyl cellulose and administered by intragastric gavage (Moon et al. 2006; Breikaa et al. 2013). G IV: infected treated with combined BCA (50 mg/kg/day) and cotrimoxazole (GlaxoSmithKline, New Cairo, Cairo, Egypt), dissolved in DW in a total dose of 370 mg/kg, divided into two doses/12 h. G V: infected treated with cotrimoxazole alone as reference drug group (370 mg/kg divided into two doses/12 h). The drug was administrated at 8 weeks post-infection for 2 weeks. All mice were euthanized by pentobarbital sodium intraperitoneal injection (14 weeks post-infection). Brain hemispheres and liver were collected, washed in saline, and stored at − 20 °C for RNA extraction. This study was approved by local Institutional Review Board Ethical Committee, Faculty of Medicine, Mansoura University (code number: R/18.12.356).
Parasitological examination
The brain specimens from all mice groups were collected. Parts of each mice brain were homogenized in 1 mL phosphate-buffered saline pH 7.2. The brain cyst number was counted in 10 μL suspension per each homogenate (Kaňková et al. 2010). The cyst number was counted in 10/HPF and the mean number was calculated for each group. The size of the cysts was measured using an ocular micrometer (Araujo et al. 1996). Mice brain homogenates containing Toxoplasma cysts were mounted on microscopic slides, fixed, and stained by Giemsa stain (Garcia 2016) (Fig. 1).
Histopathological examination
For histopathological assessment of the mice brain and liver specimens, parts of each organ was fixed in formaldehyde (10% in PBS) at pH 7.2, then processed by sectioning and hematoxylin and eosin (H & E) staining. A parasitologist and a pathologist (blinded) examined the slides. The following scoring system was used to estimate the severity of the histopathological lesions of the brain: 0: no lesion; 1: minimal lesion limited to localized perivascular cuffs with minor mononuclear cell infiltration; 2: mild lesion and local glial cell infiltration; 3: moderate lesion and focal necrosis; and 4: severe lesion, glial cell activation, and focally extensive necrosis (Tanaka et al. 2013). The scores for lesions of each area were assessed, and the total pathological score was used for data analysis. The scoring system used to estimate the severity of liver inflammatory changes was as follows: 0: no inflammatory activity; 1: minimal portal inflammation, lymphocytic infiltration, and spotty necrosis; 2: mild portal inflammation with lymphocytic infiltration and mild necrosis; 3: moderate portal inflammation, moderate lymphocytic infiltration, and noticeable hepatocellular change; and 4: severe portal inflammation, lymphocytic infiltration with possible fibrosis, and prominent diffuse hepatocellular necrosis (Batts and Ludwig 1995).
Assessment of TNF-α, IL-1β, and iNOS gene expression by real-time PCR
Brain and liver tissue samples were homogenized by five strokes of liquid nitrogen. According to the manufacturer specifications, total cellular RNA was extracted using the QIAzol reagent (Qiagen, Hilden, Germany). The yield and purity of RNA were measured by NanoDrop 2000 (Thermo Scientific, MA, USA). Reverse transcription of 1 μg of RNA was done using SensiFAST™ cDNA Synthesis Kit (Bioline, London, UK). Quantitative real-time PCR (RT-qPCR) was carried out with HERA SYBR green PCR Master Mix (Willowfort, Birmingham, UK) in a total volume of 20 μL using Pikoreal 96 instrument (Thermo Scientific, MA, USA). The amplification reaction contained a 20 μL total volume mixture [10 μL of HERA SYBR green PCR Master Mix (Willowfort, Birmingham, UK), 2 μL of cDNA template, 2 μL (10 pmol/μL) gene primers, and 6 μL of nuclease-free water]. The reaction was 95 °C for 2 min, 40 cycles of 95 °C for 10 s, 60 °C for 30 s. The sequences of the used mouse primer pairs were as follows: TNF-α forward, 5′ TGAACTTCGGGGTGATCGGT 3′, reverse, 5′ GGTGGTTTGTGAGTGTGAGGG 3′ (Ref Seq: NM_001278601.1) and the product length was 99 bp; IL-1β forward, 5′ GCAACTGTTCCTGAACTCAACT 3′, reverse, 5′ GGGTCCGTCAACTTCAAAGA 3′ (Ref Seq: NM_008361.4) and the product length was 81 bp; iNOS forward, 5′ CAGCTGGGCTGTACAAACCTT 3′, reverse, 5′ CATTGGAAGTGAAGCGTTTCG 3′ (Ref Seq: NM_001313921.1) and the product length was 95 bp; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′ AGGTCGGTGTGAACGGATTTG 3′, reverse, 5′ TGTAGACCATGTAGTTGAGGTCA 3′ (Ref Seq: NM_001289726.1) and the product length was 123 bp; GAPDH was used as the control gene (Panina et al. 2018). The primer sets were designated using Primer 3 software (v.4.1.0) [http://primer3.ut.ee], and primer specificity was determined using the Primer-BLAST program (NCBI/primer-BLAST) [https://www.ncbi.nlm.nih.gov/tools/primer-blast/]. Primer sets were ordered from Vivantis (Vivantis Technologies, Malaysia). The products were inspected visually on a 3% agarose gel with ethidium bromide staining and the data were presented as the means of 3 independent experiments. Furthermore, the data for relative fold change of gene expression were presented in terms of relative quantification (RQ) of target mRNA, normalized in respect to the housekeeping gene (GAPDH) and relative to the control sample (calibrator). The RQ of mRNA expression was calculated using the comparative threshold method (ΔΔCt) (Livak and Schmittgen 2001).
Statistical analysis
Data were analyzed using IBM-SPSS software version 20 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 8 (GraphPad Software Inc., CA, USA). Quantitative data were tested using Shapiro–Wilk’s test and expressed as mean ± SD. One-way ANOVA was used to compare data between groups followed by post hoc multiple comparisons, Tukey test. Data were considered statistically significant at P value < 0.05.
Results and discussion
Parasitological assessment of Toxoplasma cyst in the brain
As shown in Table 1, treatment with BCA (50 mg/kg/day) for 2 weeks significantly reduced the mean of T. gondii cyst count in mice brain tissues (P < 0.001). Combined BCA and cotrimoxazole significantly reduced the brain cyst count (P < 0.05). Reduction rates of 71.5%, 89.6%, and 76% were recorded in G III, G IV, and G V, respectively. No complete eradication of the cysts was achieved in any group. Cysts from both treatment groups (G III and G IV) were few and significantly smaller in size (P < 0.001) with irregular outlines than infected control mice.
A potential antimicrobial activity of BCA was reported (Dastidar et al. 2004). BCA showed anti-parasitic activity against Leishmania chagasi and Trypanosoma cruzi (Sartorelli et al. 2009). In this study, a potential anti-parasitic effect of BCA was demonstrated by significant lower toxoplasmic cyst count and size in the brain tissues. This effect could be attributed to the level of iNOS in the brain. An additional explanation could be linked to the inhibitory effect of BCA on the MAPK pathway. T. gondii possessed MAPK activity: TgMAPK-1 and TgMAPK-2 functional homologs, with a suggested role of TgMAPK-1 in parasite proliferation and stage differentiation (Brumlik et al. 2004).
Several potential mechanisms could explain BCA effect on Toxoplasma cyst count in the brain tissues. BCA was recognized to target cell cycle regulatory proteins particularly cyclin-dependent kinases and cyclins resulting in arresting cancer cellular growth (Seo et al. 2011). Its effect as COX-2 inhibitor could inhibit T. gondii proliferation (Pereira et al. 2019; Yu et al. 2019). BCA effect as MAPK kinase inhibitor could inhibit T. gondii MAPK and reduce the host cell invasion (Robert-Gangneux et al. 2000; Kole et al. 2011).
BCA selectively inhibited phosphodiesterase-4 activity with decreased intracellular Ca2 + (Ko et al. 2004). Ca2 + signaling is essential for the cellular T. gondii parasitism and replication (Hortua Triana et al. 2018). Ca2 + uptake and storage are crucially needed for the intracellular parasites as T. gondii, particularly during cellular invasion and dissemination (Vella et al. 2021).
Histopathological examination findings in the brain and liver
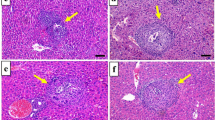
Brain sections from infected control G II showed marked inflammatory infiltrate. Severe gliosis was detected in most sections with lymphocyte infiltration and degenerated neural cells (Fig. 2b–d) compared to uninfected tissues. In both BCA- (G III) and combined-treated (G IV) mice, brain sections showed reduced inflammatory lesions compared to infected control mice, where inflammatory infiltrate ranged from mild to moderate degrees (Fig. 2e–h).
Effects of treatment regimens on the histopathological findings of brain specimens from all mice groups (H & E stained, × 200). a Normal brain tissues of uninfected mice. b–d Infected control; severe inflammatory infiltrate (black arrows), severe gliosis (white arrow), and giant cells (arrowheads). e, f Infected BCA-treated; mild to moderate inflammatory cellular infiltrates (black arrows) and mild gliosis (white arrow). g, h Infected combined-treated; g mild inflammatory cellular infiltrate (black arrows) and mild gliosis (white arrow). i, j Brain tissues of infected mice showed Toxoplasma cysts (H & E stained, × 400)
Liver tissues from infected mice showed moderate to severe inflammatory infiltrate with portal dilatation and periportal mononuclear infiltration of lymphocytes, histiocytes, and giant cells (Fig. 3b–d) compared to uninfected. In BCA- (G III) and combined-treated (G IV) mice, inflammatory infiltrate ranged from mild (60%) to moderate (40%) degrees (Fig. 3e–h). In combined-treated group, tissues of the brain and liver showed significantly reduced inflammatory scores (P < 0.001) compared to infected control (Fig. 4). In addition, in BCA group the inflammatory scores were significantly reduced (P < 0.05).
Effects of treatment regimens on the histopathological findings of liver specimens from all mice groups (H & E stained, a–d, f, h × 200; e, g × 100). a Normal liver tissue of uninfected mice. b–d Infected control, showed severe portal inflammation (black arrows), necroinflammatory injury (white arrow), and severe steatosis (arrowheads). e, f Infected BCA-treated; mild to moderate necroinflammatory injury (white arrows), mild portal inflammation (black arrow). g, h Infected combined-treated; mild portal inflammation (black arrow) and mild necroinflammatory injury (white arrow)
In this study, histopathological examination of the brain and liver tissues of BCA-treated and combined-treated mice showed milder inflammatory changes than the infected control group. Anti-inflammatory effects of BCA were demonstrated in previous studies (Kole et al. 2011; Saviranta et al. 2011; Wu et al. 2018).
Effects of BCA on TNF-α, IL-1β, and iNOS gene expression in brain and liver tissues
IL-1β expression levels were significantly increased in brain tissues of the infected mice compared to uninfected group (P < 0.001). Treatment with BCA (G III) significantly inhibited TNF-α and IL-1β expression levels (P < 0.05 and P < 0.001, respectively). However, in combined treatment G IV, no effect on TNF-α was detected compared to the infected control. The expression levels of iNOS gene were downregulated in the infected control group. Conversely, iNOS gene expression levels were significantly upregulated (P < 0.001) in BCA treatment group and in combined treatment group. The mRNA expression levels of all cytokines in the control mice treated with BCA were comparable to the untreated infected control. G V treated with cotrimoxazole showed no effect on TNF-α and iNOS mRNA levels compared to infected control G II; however, significantly reduced IL-1β mRNA levels were detected (P < 0.05) (Figs. 5 and 7a).
Effects of treatment regimens on TNF-α, IL-1β, and iNOS gene expression levels in the brain tissues of mice with chronic toxoplasmosis, analyzed by quantitative real-time PCR. G IA: uninfected control, G IB: uninfected BCA-treated. G II: infected control, G III: infected BCA-treated, G IV: infected treated with combined BCA and cotrimoxazole, G V: infected cotrimoxazole-treated. a: P < 0.05 vs. uninfected control group, b: P < 0.001 vs. uninfected group, c: P < 0.05 vs. infected control, d: P < 0.001 vs. infected control, e: P < 0.001 vs. BCA-treated, f: P < 0.05 vs. combined-treated, g: P < 0.001 vs. combined-treated
The expression levels of TNF-α and IL-1β were significantly increased in the liver tissues of infected mice (P < 0.001). Treatment with BCA alone or combined significantly decreased TNF-α and IL-1β mRNA levels in liver tissues (P < 0.001). BCA significantly inhibited the expression level of iNOS in both treatment groups (P < 0.001) (Figs. 6 and 7b). The mRNA expression levels of the cytokines in the control G 1B treated with BCA were comparable to the negative control G1A. G V treated with cotrimoxazole showed no effect on IL-1β and iNOS mRNA levels compared to infected control G II; however, significantly reduced TNF-α expression levels were detected (P < 0.05).
Effects of treatment regimens on TNF-α, IL-1β, and iNOS gene expression levels in liver tissues of mice with chronic toxoplasmosis, analyzed by quantitative real-time PCR. G IA: uninfected control, G IB: uninfected BCA-treated. G II: infected control, G III: infected BCA-treated, G IV: infected treated with combined BCA and cotrimoxazole, G V: infected cotrimoxazole-treated. a: P < 0.05 vs. uninfected control group, b: P < 0.001 vs. uninfected group, c: P < 0.05 vs. infected control, d: P < 0.001 vs. infected control, e: P < 0.001 vs. BCA-treated, f: P < 0.05 vs. combined-treated, g: P < 0.001 vs. combined-treated
Gel electrophoresis of the real-time PCR products of the studied genes in different mice groups; a in the brain tissue samples, b in the liver tissue samples. Lane 1: 50 bp ladder (L), lane 2: negative control (NC), lane 3: negative control treated with biochanin A (NC + Bio), lane 4: positive control non-treated (PC), lane 5: positive control treated with cotrimoxazole (PC + co), lane 6: positive infected treated with biochanin A (Bio), and lane 7: infected treated with combined biochanin A and cotrimoxazole (Bio + co). IL1β qPCR product (81 bp), iNOS qPCR product (95 bp), TNFα qPCR product (99 bp), and GAPDH qPCR product (123 bp)
In this study, the assessment of proinflammatory biomarkers showed that BCA treatment reduced of TNF-α and IL-1β mRNA levels. Nevertheless, it upregulated the gene expression levels of iNOS in the brain tissues of mice with chronic toxoplasmosis. In agreement with our study, Wu et al. (2015) reported that BCA significantly decreased lipopolysaccharide (LPS)-induced mRNA expression of TNF-α and IL-1β.
IFN-γ is considered a major cytokine against Toxoplasma intracellular invasion and replication (Suzuki and Remington 1988). The role of iNOS (an IFN-γ-inducible protein) and the nitric oxide production has been established in the mice to keep the dormancy of T. gondii tissue cysts (Scharton-Kersten et al. 1997).
Meira et al. (2014) assessed IFN-γ and TNF-α activity in chronic toxoplasmosis patients versus reactivated cerebral and ocular toxoplasmosis patients. Patients from reactivated groups had low levels of IFN-γ when compared with those from chronic toxoplasmosis group although they had higher TNF-α levels. The most feared complication from chronic toxoplasmosis is the reactivation of latent infection; particular attention was given to TNF in our study. High TNF-α levels were implicated in the inflammation during reactivated toxoplasmosis suggesting the significance of BCA treatment of toxoplasmosis in decreasing TNF-α without affecting IFN-γ levels.
A study conducted on experimental bronchial asthma murine model showed that BCA suppressed the levels of cytokines, including TNF-α in the lung broncho-alveolar lavage fluid. However, it did not affect IFN-γ level (Ko et al. 2011).
Upregulation of proinflammatory cytokines expression, including iNOS, plays a major role in antibacterial response mechanisms. However, excessive production has been implicated in the impairment of the anti-oxidative system and exaggerated pathological inflammatory reactions (Matsumoto et al. 1998).
The iNOS expression levels were found to be downregulated in experimental parasitic infection with Leishmania donovani, an intracellular protozoan (Kole et al. 1999) and Trichinella spiralis nematode (Bian et al. 2001), which agreed with our findings. Our study showed that infection with T. gondii downregulated iNOS levels in infected control mice group. However, BCA treatment resulted in upregulation of iNOS gene expression levels in the brain with a significant decline in Toxoplasma cyst count.
According to Kole et al. (1999), doxorubicin-containing liposome combined with IFN-γ treatment of mice model infected Leishmania donovani resulted in upregulation of iNOS expression levels and subsequent reducing parasite numbers.
A high parasitic burden of Toxoplasma tissue cysts was found in the brain and might occupy up to 92% of the brain tissue (Berenreiterová et al. 2011). In addition, inflammatory infiltrates and foci of necrosis were observed independently of the Toxoplasma cyst sites.
The iNOS inhibitors could not reactivate chronic cerebral toxoplasmosis in BALB/c mice, but caused reactivation of chronic toxoplasmic encephalitis with a significantly increased intracerebral cyst count in T. gondii susceptible C57BL/6 mice (Schlüter et al. 1999). Low levels of iNOS expression were demonstrated in the tissues loaded with Toxoplasma cysts in mice infected with chronic toxoplasmosis, suggesting that histopathological lesions in organs were associated with parasite load rather than the level of iNOS (Schlüter et al. 1999). Previous findings agreed with our results. In our study, the histopathological scores in the brain were significantly lower in treated mice, with increased iNOS expression levels, than the non-treated mice.
In the present study, BCA treatment in chronic toxoplasmosis achieved a dual action: the suppression of TNF-α and IL-1β as proinflammatory cytokines, and a concomitant induction iNOS synthase. Increased iNOS expression levels in chronically infected mice with toxoplasmosis could be linked to the anti-parasitic activity of BCA. In another study, BCA inhibited both inflammatory and anti-inflammatory reactions in a Salmonella-infected mice model (Zhao et al. 2018).
Our data showed that BCA significantly inhibited TNF-α, IL-1β, and iNOS mRNA expression levels in the liver tissues. While Toxoplasma cysts are primarily present in the brain tissues during chronicity, parasitic load in the liver is far inferior to the brain burden at the eighth week post-infection (Autier et al. 2018). This could explain the controversial effects on iNOS expression, as BCA downregulates mRNA expression of iNOS in the liver tissues of mice with chronic toxoplasmosis. Anti-inflammatory effects of BCA were observed notably in liver tissues in this study. An interaction between hepatic stellate cells and T. gondii antigens was suggested to play a role in the liver pathology of toxoplasmosis-related hepatitis (Atmaca et al. 2013). Administration of BCA has been found to counteract hepatic damage caused by arsenic (Jalaludeen et al. 2016).
The combination of BCA and other drug therapies was assumed to be safe with a low possibility of alterations in the pharmacokinetics of the co-administered drugs (Arora et al. 2015). In our study, treatment with BCA alone downregulated TNF-α and IL-1β mRNA expression in the brain, while combined treatment resulted in higher levels. This could be attributed to cotrimoxazole effects that hindered back anti-inflammatory effects of BCA. Cotrimoxazole (trimethoprim/sulfamethoxazole) had a history of drug-drug interactions resulting in synergistic or antagonistic outcomes (Kaysadu et al. 2019). In this study, treatment with cotrimoxazole alone showed mild variable effects on the brain and liver tissue cytokines. According to Dubar et al. (1990), no significant effect was demonstrated by cotrimoxazole on IL-1 and TNF production, which agreed mostly with our findings.
In conclusion, the present study suggests potential anti-inflammatory effects of biochanin A in experimental chronic toxoplasmosis. These effects were demonstrated by attenuation of the pathological lesions in mice brain and liver tissues. Upregulation of iNOS expression levels in the mice brain tissues, associated with a significant decline in the parasitic cyst load, was also detected.
References
Araujo FG, Suzuki Y, Remington JS (1996) Use of rifabutin in combination with atovaquone, clindamycin, pyrimethamine, or sulfadiazine for treatment of toxoplasmic encephalitis in mice. Eur J Clin Microbiol Infect Dis 15(5):394–397. https://doi.org/10.1007/BF01690096
Arora S, Taneja I, Challagundla M, Raju KS, Singh SP, Wahajuddin M (2015) In vivo prediction of CYP-mediated metabolic interaction potential of formononetin and biochanin A using in vitro human and rat CYP450 inhibition data. Toxicol Lett 239:1–8. https://doi.org/10.1016/j.toxlet.2015.08.202
Atmaca HT, Gazyagcı AN, Canpolat S, Kul O (2013) Hepatic stellate cells increase in Toxoplasma gondii infection in mice. Parasit Vectors 6:135. https://doi.org/10.1186/1756-3305-6-135
Autier B, Dion S, Robert-Gangneux F (2018) The liver as an organ at risk for Toxoplasma transmission during transplantation myth or reality? J Clin Pathol 71:763–766. https://doi.org/10.1136/jclinpath-2018-205066
Batts T, Ludwig J (1995) Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 19:1409–1419
Berenreiterová M, Flegr J, Kuběna AA, Němec P (2011) The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS ONE 6:e28925. https://doi.org/10.1371/journal.pone.0028925
Bhardwaj V, Tadinada SM, Jain A, Sehdev V, Daniels CK, Lai JC, Bhushan A (2014) Biochanin A reduces pancreatic cancer survival and progression. Anticancer Drugs 25:296–302. https://doi.org/10.1097/CAD.0000000000000044
Bian K, Harari Y, Zhong M, Lai M, Castro G, Weisbrodt N, Murad F (2001) Down-regulation of inducible nitric-oxide synthase (NOS-2) during parasite-induced gut inflammation: a path to identify a selective NOS-2 inhibitor. Mol Pharmacol 59:939–947. https://doi.org/10.1124/mol.59.4.939
Breikaa RM, Algandaby MM, El-Demerdash E, Abdel-Naim AB (2013) Multimechanistic antifibrotic effect of biochanin a in rats: implications of proinflammatory and profibrogenic mediators. PLoS ONE 8:e69276. https://doi.org/10.1371/journal.pone.0069276
Brumlik MJ, Wei S, Finstad K, Nesbit J, Hyman LE, Lacey M, Burow ME, Curiel TJ (2004) Identification of a novel mitogen-activated protein kinase in Toxoplasma gondii. Int J Parasitol 34:1245–1254. https://doi.org/10.1016/j.ijpara.2004.07.007
Dastidar SG, Manna A, Kumar KA, Mazumdar K, Dutta NK, Chakrabarty AN et al (2004) Studies on the antibacterial potentiality of isoflavones. Int J Antimicrob Agents 23:99–102. https://doi.org/10.1016/j.ijantimicag.2003.06.003
Denkers EY (1999) T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect 1:699–708. https://doi.org/10.1016/s1286-4579(99)80071-9
Dubar V, Lopez I, Gosset Ph, Aerts C, Voisin C, Wallaert B (1990) The penetration of co-trimoxazole into alveolar macrophages and its effect on inflammatory and immunoregulatory functions. J Antimicrob Chemother 26:791–802
Dunn AJ (2006) Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res 6:52–68. https://doi.org/10.1016/j.cnr.2006.04.002
Garcia LS (2016) Procedures for detecting blood parasites. In: Garcia LS (ed) Diagnostic medical parasitology, 6th edn. ASM Press, Washington, pp 136–137
Hortua Triana MA, Márquez-Nogueras KM, Vella SA (1865) Moreno SNJ (2018) Calcium signaling and the lytic cycle of the apicomplexan parasite Toxoplasma gondii. Biochim Biophys Acta Mol Cell Res 1865(11 Pt B):1846–1856. https://doi.org/10.1016/j.bbamcr.2018.08.004
Jalaludeen AM, Ha WT, Lee R, Kim JH, Do JT, Park C et al (2016) Biochanin A ameliorates arsenic-induced hepato and hematotoxicity in rats. Molecules 21:69. https://doi.org/10.3390/molecules21010069
Kaňková S, Holáň V, Zajícová A, Kodym P, Flegr J (2010) Modulation of immunity in mice with latent toxoplasmosis—the experimental support for the immunosuppression hypothesis of Toxoplasma-induced changes in reproduction of mice and humans. Parasitol Res 107:1421–1427
Kaysadu H, Duman Y, Yakupogulları Y (2019) Investigation of synergistic, additive and antagonist effect of antimicrobial combinations used for Brucella spp, with E-test combination method. Eastern J Med 24:96–101. https://doi.org/10.5505/ejm.2019.66588
Kim L, Del Rio L, Butcher BA, Mogensen TH, Paludan SR, Flavell RA, Denkers EY (2005) p38 MAPK autophosphorylation drives macrophage IL-12 production during intracellular infection. J Immunol 174:4178–4184. https://doi.org/10.4049/jimmunol.174.7.4178
Ko WC, Shih CM, Lai YH, Chen JH, Huang HL (2004) Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem Pharmacol 68(10):2087–2094. https://doi.org/10.1016/j.bcp.2004.06.030
Ko WC, Lin LH, Shen HY, Lai CY, Chen CM, Shih CH (2011) Biochanin a, a phytoestrogenic isoflavone with selective inhibition of phosphodiesterase 4, suppresses ovalbumin-induced airway hyperresponsiveness. Evid Based Complement Alternat Med 2011:635058. https://doi.org/10.1155/2011/635058
Kole L, Das L, Das PK (1999) Synergistic effect of interferon-gamma and mannosylated liposome-incorporated doxorubicin in the therapy of experimental visceral leishmaniasis. J Infect Dis 180:811–820. https://doi.org/10.1086/314929
Kole L, Giri B, Manna SK, Pal B, Ghosh S (2011) Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur J Pharmacol 653:8–15. https://doi.org/10.1016/j.ejphar.2010.11.026
Lam AN, Demasi M, James MJ, Husband AJ, Walker C (2004) Effect of red clover isoflavones on cox-2 activity in murine and human monocyte/macrophage cells. Nutr Cancer 49:89–93. https://doi.org/10.1207/s15327914nc4901_12
Lang D, Schott BH, van Ham M, Morton L, Kulikovskaja L, Herrera-Molina R et al (2018) Chronic Toxoplasma infection is associated with distinct alterations in the synaptic protein composition. J Neuroinflammation 15:216. https://doi.org/10.1186/s12974-018-1242-1
Liesenfeld O, Parvanova I, Zerrahn J, Han SJ, Heinrich F, Muñoz M et al (2011) The IFN-γ-inducible GTPase, Irga6, protects mice against Toxoplasma gondii but not against Plasmodium berghei and some other intracellular pathogens. PLoS ONE 6:e20568. https://doi.org/10.1371/journal.pone.0020568
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lu L, Zhang X, Tong H, Zhang W, Xu P, Qu S (2017) Central administration of 5Z–7-oxozeaenol protects experimental autoimmune encephalomyelitis mice by inhibiting microglia activation. Front Pharmacol 8:789. https://doi.org/10.3389/fphar.2017.00789
Mahmoudvand H, Ziaali N, Ghazvini H, Shojaee S, Keshavarz H, Esmaeilpour K, Sheibani V (2016) Toxoplasma gondii infection promotes neuroinflammation through cytokine networks and induced hyperalgesia in BALB/c mice. Inflammation 39:405–412. https://doi.org/10.1007/s10753-015-0262-6
Matsumoto S, Okabe Y, Setoyama H, Takayama K, Ohtsuka J, Funahashi H, Imaoka A, Okada Y, Umesaki Y (1998) Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut 43:71–78. https://doi.org/10.1136/gut.43.1.71
McCusker RH, Kelley KW (2013) Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol 216:84–98. https://doi.org/10.1242/jeb.073411
Meira CS, Pereira-Chioccola VL, Vidal JE, de Mattos CC, Motoie G, Costa-Silva TA et al (2014) Cerebral and ocular toxoplasmosis related with IFN-γ, TNF-α, and IL-10 levels. Front Microbiol 5:492. https://doi.org/10.3389/fmicb.2014.00492
Moon YJ, Sagawa K, Frederick K, Zhang S, Morris ME (2006) Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J 8:E433–E442. https://doi.org/10.1208/aapsj080351
Nogueira PM, Ribeiro K, Silveira AC, Campos JH, Martins-Filho OA, Bela SR et al (2015) Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J Extracell Vesicles 4:28734. https://doi.org/10.3402/jev.v4.28734
Panina Y, Germond A, Masui S, Watanabe TM (2018) Validation of common housekeeping genes as reference for qPCR gene expression analysis during iPS reprogramming process. Sci Rep 8:8716. https://doi.org/10.1038/s41598-018-26707-8
Pappas G, Roussos N, Falagas ME (2009) Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol 39:1385–1394. https://doi.org/10.1016/j.ijpara.2009.04.003
Pereira ACA, Silva RJ, Franco PS, de Oliveira GA, Souza G, Milian ICB et al (2019) Cyclooxygenase (COX)-2 inhibitors reduce Toxoplasma gondii infection and upregulate the pro-inflammatory immune response in Calomys callosus rodents and human monocyte cell line. Front Microbiol 10:225. https://doi.org/10.3389/fmicb.2019.00225
Robert-Gangneux F, Creuzet C, Dupouy-Camet J, Roisin MP (2000) Involvement of the mitogen-activated protein (MAP) kinase signalling pathway in host cell invasion by Toxoplasma gondii. Parasite 7(2):95–101. https://doi.org/10.1051/parasite/2000072095
Sartorelli P, Carvalho CS, Reimão JQ, Ferreira MJ, Tempone AG (2009) Antiparasitic activity of biochanin A, an isolated isoflavone from fruits of Cassia fistula (Leguminosae). Parasitol Res 104:311–314. https://doi.org/10.1007/s00436-008-1193-z
Saviranta NMM, Veeroos L, Granlund LJ, Hassinen VH, Kaarniranta K, Karjalainen RO (2011) Plant flavonol quercetin and isoflavone biochanin A differentially induce protection against oxidative stress and inflammation in ARPE-19 cells. Food Res Int 44:109–113. https://doi.org/10.1016/j.foodres.2010.10.056
Scharton-Kersten TM, Yap G, Magram J, Sher A (1997) Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185:1261–1273. https://doi.org/10.1084/jem.185.7.1261
Schlüter D, Deckert-Schlüter M, Lorenz E, Meyer T, Röllinghoff M, Bogdan C (1999) Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J Immunol 162:3512–3518
Schlüter D, Meyer T, Strack A, Reiter S, Kretschmar M, Wiestler OD et al (2001) Regulation of microglia by CD4+ and CD8+ T cells: selective analysis in CD45-congenic normal and Toxoplasma gondii-infected bone marrow chimeras. Brain Pathol 11:44–55. https://doi.org/10.1111/j.1750-3639.2001.tb00380.x
Seo YJ, Kim BS, Chun SY, Park YK, Kang KS, Kwon TG (2011) Apoptotic effects of genistein, biochanin-A and apigenin on LNCaP and PC-3 cells by p21 through transcriptional inhibition of polo-like kinase-1. J Korean Med Sci 26(11):1489–1494. https://doi.org/10.3346/jkms.2011.26.11.1489
Silva NM, Vieira JC, Carneiro CM, Tafuri WL (2009) Toxoplasma gondii: the role of IFN-gamma, TNFRp55 and iNOS in inflammatory changes during infection. Exp Parasitol 123:65–72. https://doi.org/10.1016/j.exppara.2009.05.011
Suzuki Y, Remington JS (1988) Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol 140:3943–3946
Tanaka S, Nishimura M, Ihara F, Yamagishi J, Suzuki Y, Nishikawa Y (2013) Transcriptome analysis of mouse brain infected with Toxoplasma gondii. Infect Immun 81:3609–3619. https://doi.org/10.1128/IAI.00439-13
Valère A, Garnotel R, Villena I, Guenounou M, Pinon JM, Aubert D (2003) Activation of the cellular mitogen-activated protein kinase pathways ERK, P38 and JNK during Toxoplasma gondii invasion. Parasite 10:59–64. https://doi.org/10.1051/parasite/2003101p59
Vella SA, Moore CA, Li ZH, Hortua Triana MA, Potapenko E, Moreno SNJ (2021) The role of potassium and host calcium signaling in Toxoplasma gondii egress. Cell Calcium 94:102337. https://doi.org/10.1016/j.ceca.2020.102337
Wu LY, Ye ZN, Zhuang Z, Gao Y, Tang C, Zhou CH et al (2018) Biochanin A reduces inflammatory injury and neuronal apoptosis following subarachnoid hemorrhage via suppression of the TLRs/TIRAP/MyD88/NF-κB pathway. Behav Neurol 2018:1960106. https://doi.org/10.1155/2018/1960106
Wu WY, Wu YY, Huang H, He C, Li WZ, Wang HL et al (2015) Biochanin A attenuates LPS-induced pro-inflammatory responses and inhibits the activation of the MAPK pathway in BV2 microglial cells. Int J Mol Med 35:391–398. https://doi.org/10.3892/ijmm.2014.2020
Yu C, Zhang P, Lou L, Wang Y (2019) Perspectives regarding the role of biochanin A in humans. Front Pharmacol 10:793. https://doi.org/10.3389/fphar.2019.00793
Zhao X, Tang X, Guo N, An Y, Chen X, Shi C et al (2018) Biochanin a enhances the defense against Salmonella enterica infection through AMPK/ULK1/mTOR-mediated autophagy and extracellular traps and reversing SPI-1-dependent macrophage (MΦ) M2 polarization. Front Cell Infect Microbiol 8:318. https://doi.org/10.3389/fcimb.2018.00318
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Dana Mordue
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aboukamar, W.A., Elhenawy, A.A., Elmehankar, M.S. et al. Activity of isoflavone biochanin A in chronic experimental toxoplasmosis: impact on inflammation. Parasitol Res 121, 2405–2414 (2022). https://doi.org/10.1007/s00436-022-07571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07571-y