Abstract
Reports on metronidazole resistance of Trichomonas vaginalis strains have been on the increase. This study investigated the in vitro metronidazole resistance patterns in T. vaginalis isolates obtained from South African pregnant women and the genotypes of these isolates. This study included 362 pregnant women recruited from a hospital in Durban, South Africa. The women provided self-collected vaginal swabs for the detection of T. vaginalis by culture in Diamonds media. Cultured isolates were then subjected to anaerobic susceptibility assays to metronidazole. For the genotyping assays, the actin gene was digested by HindII, MseI, and RsaI. The banding patterns obtained after digestion was used to determine the genotypes. A total of 21/362 (5.8%) pregnant women tested positive for T. vaginalis infection. Of the 21 T. vaginalis isolates tested for metronidazole susceptibility, 9.5% (2/21) had a minimum inhibitory concentration (MIC) of 4 μg/ml (resistant), 38.1% (8/21) had a MIC of 2 μg/ml (intermediate), and 52.4% (11/21) had a MIC ≤ 1 μg/ml (susceptible). The dominant genotype that was identified across the isolates was genotype G. There was no correlation between genotype harboured and metronidazole susceptibility patterns. In this study, resistance to metronidazole was observed in clinical isolates of T. vaginalis. This study did not find a correlation between genotype harboured and metronidazole susceptibility patterns. Despite the lack of association, our study provides data on an area of research that is currently lacking in our setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichomonas vaginalis is an anaerobic parasitic protozoan that causes the sexually transmitted infection (STI) trichomoniasis (Akbari and Matini 2017; Ramjee et al. 2015). In sub-Saharan Africa, about 30 million infections occur annually (Naidoo and Wand 2013). Studies that have been conducted in South African pregnant women have reported prevalence rates of 15.3% (Moodley et al. 2015) and 20.2% (Morikawa et al. 2018). Trichomoniasis has been found to be associated with various health complications including pelvic inflammatory disease (PID), significant pregnancy complications, cervical cancer prostatitis, and infertility (Johnston and Mabey 2008; Swygard et al. 2004). Significant pregnancy complications include pre-term labour, low birth weight, and premature rapture of membranes (Cornelius et al. 2012; Glehn et al. 2017).
Metronidazole and tinidazole are the only drugs approved by the United States Food and Drug Administration for trichomoniasis (Bradic et al. 2017). The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) treatment guidelines of trichomoniasis include metronidazole or tinidazole 2 g single dose as the recommended regimens and metronidazole 500 mg, twice a day for 7-day dose as the alternative treatment regimen. Treatment with 2 g metronidazole is recommended at any time during pregnancy (Kissinger 2015a; Kissinger 2015b). Tinidazole has not been evaluated for use during pregnancy (Kissinger 2015b; Mielczarek and Blaszkowska 2015). Metronidazole resistance has been reported in clinical isolates since 1959 (Bradic et al. 2017). Most T. vaginalis isolates are highly susceptible to metronidazole; however, laboratory resistance and treatment failure have been reported (Bosserman et al. 2011; Kirkcaldy et al. 2012). Two pathways of metronidazole resistance have been suggested which are aerobic and anaerobic resistance. Aerobic (clinical) resistance is observed in the presence of oxygen in which oxygen stress response genes might be involved in this type of resistance. In aerobic resistance, nitroradical anions are re-oxidised before they can impair the trichomonad; thus, metronidazole toxicity is reduced (Bouchemal et al. 2017; Sood and Kapil 2008). Anaerobic resistance has been observed in vitro. In the anaerobic resistant strains, the hydrogenosome enzymes (PFO and ferredoxin) and cytosolic thioredoxin reductase are absent. These enzymes are important for metronidazole activation (Bradic et al. 2017). Mutations in the nitroreductase genes of T. vaginalis have also been implicated in metronidazole resistance (Alessio and Nyirjesy 2019).
The genetic characterisation of T. vaginalis isolates from various regions shows that there is significant genetic diversity in this organism (Meade and Carlton 2013), thereby impeding vaccine development. T. vaginalis is said to contain an actin protein which is coded by a 9-membered family, having nucleotide resemblances in 5 genes which are similar to mammalian actin genes. When T. vaginalis attaches to the surface of the host cell, it causes the parasite to transform from a tetanus form to amoebic form resulting in appendage formation, i.e. pseudopodia (Shahraki et al. 2020). Both attachment and deformation regarding the host cell can be associated with the pathogenicity of T. vaginalis. The actin gene thus plays a significant role in the parasite’s pathogenicity, thereby making it the gene of choice for molecular typing techniques (Rezaeian et al. 2009).
To date, there is a lack of data on the distribution of genotypes in relation to metronidazole susceptibility patterns for pregnant women in our setting, thus the rationale for this study. This study investigated the in vitro metronidazole resistance patterns in T. vaginalis isolates obtained from South African pregnant women, and the genotypes of these isolates were determined by restriction digestion profiles of the actin gene.
Materials and methods
Study participants recruitment
This was a cross-sectional study which included 362 pregnant women recruited from the King Edward VIII hospital antenatal clinic in Durban, South Africa, during October 2018–March 2019. The women who were enrolled in the study were 18 years and older, willing to provide written informed consent and willing to provide a self-collected vaginal swab sample to be tested for T. vaginalis infection. Laboratory testing was conducted at the School of Clinical Medicine Research Laboratory at the Nelson R. Mandela School of Medicine, University of KwaZulu-Natal. All women presenting with symptoms of vaginal discharge syndrome were treated using the syndromic approach.
Specimen collection
Women were educated on self-collection of vaginal swabs (sterile individually wrapped Dacron swab). The collected vaginal swabs were immediately inoculated into a 15-ml sterile tube containing Diamond’s TYM medium (Diamond 1957). Diamond’s TYM medium was supplemented with amikacin (4μg/ml), amphotericin B (5μg/ml), ampicillin (1mg/ml), chloramphenicol (1μg/ml), ciprofloxacin (2μg/ml), and vancomycin (5μg/ml). The tubes were inoculated with the vaginal swabs at the clinic and then transported within 1 h of inoculation to the School of Clinical Medicine Research Laboratory, University of KwaZulu-Natal, for processing.
Diagnosis and propagation of T. vaginalis clinical isolates
Upon arrival at the laboratory, the culture tubes were incubated at 37°C for 7 days. Cultures were examined daily from day 2 to 7 by wet mount microscopy. A specimen was considered positive for T. vaginalis infection if cells with a jerk motility were observed. A sample was considered negative if no trophozoites were observed at day 7. A sub-culture of positive samples was performed by transferring 500μl of the culture into 5ml fresh Diamond’s TYM medium supplemented with amikacin, amphotericin B, ampicillin, chloramphenicol, ciprofloxacin, and vancomycin at 48-h intervals until non-contaminated axenic cultures were obtained. Once axenic cultures were obtained, metronidazole susceptibility assays and DNA extraction were performed.
Metronidazole susceptibility assay
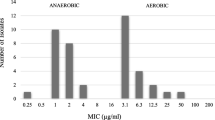
Metronidazole susceptibility was performed in 96 well flat-bottomed microtiter plates under anaerobic incubation conditions. Twofold serial dilutions of metronidazole were performed in Diamond’s TYM medium. The resulting concentrations ranged from 0.25 to 16 μg/ml. T. vaginalis cultures were then standardised to an inoculum of 1.5×104 trichomonads/well. Each T. vaginalis isolate inoculum was then added into each well excluding the ATCC control wells. The T. vaginalis ATCC 50148 strain was used as a control strain and untreated cultures of the respective isolates were used as growth controls. Plates were incubated in air-tight anaerobic jars containing Oxoid™ AnaeroGen™ 2.5L gas pack (ThermoFisher Scientific, USA) and Oxoid™ Resazurin Anaerobic indicator strip (ThermoFisher Scientific, USA) at 37°C for 48 h. T. vaginalis motility and growth were assessed using the inverted microscope at ×400 magnification. T. vaginalis growth and motility were scored according to the scoring criteria described by Upcroft (Upcroft and Upcroft 2001). Trophozoite numbers were scored 1+ (0–10 motile parasites; not more than 20% coverage of well surface and significantly less active), 2+ (20 to 50% coverage of the well surface and some trophozoite motility), 3+ (more than 50 % coverage of the well surface, almost confluent growth with much motility), and 4+ (confluent growth with full motility) (Upcroft and Upcroft 2001). The minimum inhibitory concentration (MIC) was defined as the lowest concentration of metronidazole in which a score of 1+ was observed after 48 h of incubation. Breakpoints suggested by Upcroft were used (Upcroft and Upcroft 2001). MIC ≤ 1 μg/ml was considered susceptible, MIC = 2 μg/ml was considered intermediate (low-level resistance), and MIC ≥ 4 μg/ml was considered resistant (Upcroft and Upcroft 2001). All experiments were performed in triplicate for each T. vaginalis isolate.
DNA extraction from T. vaginalis isolates
T. vaginalis DNA was extracted using the phenol-chloroform method (Shaio et al. 1997). Briefly, T. vaginalis cells were washed twice in phosphate-buffered saline (pH 7.4) by centrifugation at 1500×g for 10 min. DNA was extracted from the cell pellets by adding 500 μl of lysis buffer and incubated at 65°C for 30 min. The lysis buffer was prepared from concentrated stock solutions to obtain final concentrations of 100 μg of proteinase K, 450 mM NaCl, 15 mM sodium citrate, and 0.2% sodium dodecyl sulphate (SDS) per ml. The extracted DNA was then purified twice by adding an equal volume of phenol-chloroform (1:1; vol/vol) and centrifuged at 1500×g for 10 min. DNA was then purified once with chloroform only. DNA extracts were then precipitated with 2 volumes of 95% ethanol (vol/vol) and 0.1 volume of 3 M sodium acetate (pH 5.2). The DNA pellets were then washed with 70% (vol/vol) ethanol, air-dried at room temperature, and dissolved in 50 μl of TE buffer. The concentration and purity of the extracted DNA were measured using the NanoDrop Spectrophotometer (ThermoFisher Scientific, USA).
Confirmation of T. vaginalis by 18S ribosomal RNA PCR
A forward primer S1 (5′-TCCCGGATAATTGAAACGGA-3′) and a reverse primer S2 (5′-GAATGTGATAGCGAAATGGG-3′) were used to amplify a region of approximately 413-bp within the 650-bp repeat region. PCR was performed in a total volume of 50 μl. The reaction mixture for each T. vaginalis isolate contained 16 μl of nuclease-free PCR water, 25 μl of the DreamTaq PCR Master Mix (ThermoFisher Scientific, United States), 2 μl of each primer, and 5 μl template DNA. The cycling conditions were initial denaturation at 94°C for 3 min followed by 40 cycles at 94°C for 45 s, 50°C for 40 s, 72°C for 1 min, and a final extension at 72°C for 7 min. PCR amplification was performed in a T100 thermocycler (BioRad, USA). PCR products were analysed by electrophoresis on a 1% agarose gel in 0.5X TBE buffer at 80 V and viewed under a UV illumination (Gene Genius System).
Detection of the actin genes from T. vaginalis
A conventional nested PCR assay was used for the amplification of the actin genes (outer and inner regions) using oligonucleotide primers, published by Espinosa et al. (Espinosa et al. 2001) and Khalili et al. (Khalili et al. 2017). The primers used for amplification of inner and outer actin genes are shown in Table 1.
Amplification of the outer actin gene
The PCR amplification reactions were performed with a total volume of 25 μl. The reaction contained 12.5 μl DreamTaq master mix (ThermoFisher Scientific, USA), 9.5 μl distilled water, and 0.5 μl of each primer (reverse and forward), and 2 μl of template DNA was used. The negative control contained 23 μl of PCR mixture and 2 μl of distilled water. Thereafter, the PCR tubes were placed into the thermal cycler, and the following conditions was performed, for gene amplification initial denaturation at 94°C for 5 min, thereafter 30 cycles: denaturation at 94°C for 1 min, annealing 54°C for 1 min, elongation 72°C for 1 min, and final elongation at 72°C for 5 min.
Amplification of the inner actin gene by nested PCR
The nested amplification reactions were performed with a total volume of 25 μl. The reaction contained 12.5 μl DreamTaq master mix, 9.5 μl distilled water, 0.5 μl of each primer (reverse and forward), and 2 μl of outer PCR product. The negative control contained 23 μl of PCR mixture and 2 μl of distilled water. Thereafter, the PCR tubes was placed into the thermal cycler, and the following conditions was performed, for gene amplification initial denaturation at 94°C for 5 min, thereafter 30 cycles: denaturation at 94°C for 1 min, annealing 45°C for 1 min, elongation 72°C for 1 min, and final elongation at 72°C for 5 min.
Sequence confirmation of the actin gene
A subset of PCR positive amplicons was sequenced to confirm the presence of the gene prior to the genotyping analysis. Sanger DNA sequencing was performed on the inner actin PCR amplicons. Each amplicon was sequenced in both directions to cover the full-length actin gene. The sequencing was conducted using the BrilliantDye™ Terminator v3.1 Cycle Sequencing on an ABI3500XL genetic analyser. The sequencing was performed at Inqaba Biotechnical Industries (Hatfield, Pretoria, South Africa). The ABI sequencing files were edited on CHROMAS (Technelysium, Queensland, Australia). The forward and reverse sequences were aligned using the DNAMAN software (Lynnon Biosoft, CA, USA). The identity of the edited sequences was confirmed using the National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST).
Restriction fragment length polymorphisms (RFLP)
The genotyping of the actin gene was performed using the RFLP technique. Restriction enzymes, HindII, MseI, and RsaI, were used to generate the banding profiles. The inner actin amplicons were digested with the individual enzymes. The digestion mix was made up to a final volume of 20 μl. Each reaction consisted of 0.5 μl enzyme, 2 μl enzyme buffer, 0.2 μl bovine serum albumin (BSA), 7.3 μl distilled water, and 10 μl of the PCR amplicon. The digestion reactions were incubated for 1 h under the following temperature conditions: 37°C for all enzymes followed by heat inactivation at 65°C for 20 min for HindII and MseI. Following incubation, the digests were run on a 2% agarose gel. The enzymes banding patterns and assignment of genotypes based on a composite of the patterns was determined according to Khalili et al. (Khalili et al. 2017).
Results
Prevalence and metronidazole susceptibility patterns
A total of 21/362 (5.8%) pregnant women tested positive for T. vaginalis infection. The median interquartile range (Q1–Q3) age of the women who tested positive for T. vaginalis infection was 27.0 (24.0–36.0). The 413bp fragment corresponding to the 18S rRNA gene from T. vaginalis was present in all 21 samples amplified and confirmed by DNA sequencing (100% identity to Trichomonas vaginalis G3, Accession number: XM_001292180.1).
Anaerobic metronidazole MICs ranged from 0.25 to 4 μg/ml, and the mean MICs ± standard deviation was 1.63 ± 0.95. Of the 21 T. vaginalis isolates tested for anaerobic metronidazole susceptibility, 9.5% (2/21) had a MIC of 4 μg/ml (resistant), 38.1% (8/21) had a MIC of 2 μg/ml (intermediate), and 52.4% (11/21) had a MIC ≤ 1 μg/ml (susceptible) (Table 2). The MIC of the T. vaginalis ATCC 50148 control strain was 1μg/ml which was within the expected range (Müller et al. 1988).
Genotypes detected
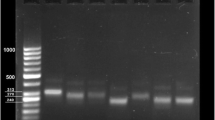
The DNA sequencing hits of the actin PCR amplicons showed identity (99%) to T. vaginalis isolate 19 actin gene (MF350343.1) and T. vaginalis strain ATCC 30240 actin gene (99%) (EU076579.1). Of the 21 isolates investigated in this study, 4 samples did not produce genotyping profiles and were excluded from further analysis. When digested with HindII, all samples produced the same banding profile and bands at positions 213bp, 300bp, 300bp, and 426bp (Fig. 1). Based on the banding profile obtained, the isolates were assigned pattern 2. Figure 2 shows the banding profile of selected isolates after digestion with MseI. Similar to the HindII digests, the same banding profile was observed for all isolates. Band sizes of 519bp and 581bp were obtained after digestion which indicated banding pattern 1 (Fig. 2). For the RsaI digestion, one isolate had produced a different banding prolife from the other isolates. The isolate 341 had produced band sizes of 106bp, 190bp, 300bp, and 452bp. This isolate was assigned pattern 3. The remaining isolates had produced band sizes of 106bp, 250bp, and 586bp and were assigned pattern 1 (Fig. 3).
Based on the patterns generated, genotypes were assigned. Table 3 describes the assignment of the genotypes based on banding patterns. Of the 17 isolates, 16 isolates (94.1%) harboured genotype G, and the one isolate harboured genotype I. The distribution of the genotypes across the MIC profiles is shown in Table 4. According to Table 4, the I genotype was carried by an intermediate isolate, and the G genotype was distributed across susceptible, intermediate, and resistant isolates. There was no correlation between genotype harboured and metronidazole susceptibility patterns.
Discussion
Trichomoniasis is the most common STI with an estimated annual incidence of 276.4 million cases globally (Akbari and Matini 2017; Bouchemal et al. 2017; Gatti et al. 2017; Ramjee et al. 2015). To the best of our knowledge, this study was the first to investigate the in vitro metronidazole resistance patterns in T. vaginalis isolates obtained from South African pregnant women and the correlation between the genotypes and drug susceptibility patterns.
Reports on metronidazole resistance of T. vaginalis strains have been increasing (Ramjee et al. 2015). Even though the metronidazole cure rates are high, clinical treatment failure is challenging (Petrin et al. 1998). Most T. vaginalis isolates are highly susceptible to metronidazole; however, laboratory resistance and treatment failure have been reported (Bosserman et al. 2011; Kirkcaldy et al. 2012). Approximately 2.5 to 10% of T. vaginalis isolates are resistant to metronidazole treatment (Kusdian and Gould 2014). In this study, 9.5% of the isolates produced resistant profiles towards metronidazole with the majority of the isolates being susceptible. In South Africa, metronidazole resistance was reported in 6% of T. vaginalis strains isolated from women attending an antiretroviral clinic (Rukasha et al. 2013). A study conducted by Abdel-Magied et al. (Abdel-Magied et al. 2017) reported a prevalence of 8.2% for metronidazole resistance in non-pregnant women from Egypt. This study builds on the data by Mabaso et al. (2020) who have reported on metronidazole resistance in pregnant women, and now the current study shows the association between resistance profiles and prevalent genotypes.
The genetic characterisation of T. vaginalis isolates from various regions show that there is significant genetic diversity in this organism (Meade and Carlton 2013). In this study, genotype G was shown to be the most prevalent in the isolates investigated. Only a single isolate carried genotype I. Our findings of the dominant genotype G have also been reported for other populations. A study conducted in Zambia on female sex workers revealed the presence of eight different genotypes based on the actin gene, the most common genotype was G (Crucitti et al. 2008). In another study conducted in Ndola, Zambia, involving adolescent girls, pregnant women as well as sex workers, Crucitti et al. (2010) identified nine different genotypes, with genotype G being the most frequent across all three study groups. A study conducted by Momeni et al. (Momeni et al. 2015) identified five different genotypes with genotype G being the most prevalent in a population of Iranian men and women.
In this study, based on the high dominance of genotype G (94.1%) in the isolates tested, there was no observed correlation between genotypes and metronidazole resistance patterns. In addition, there is a lack of data on the correlation of drug susceptibility patterns in relation to genotyping based on the actin gene. Our study now provides data on this correlation. Past studies have found significant associations between drug susceptibility patterns and genotypes based on other gene targets. A study conducted by Abdel-Magied et al. (Abdel-Magied et al. 2017) reported on a correlation between metronidazole susceptibility patterns and genotypes of T. vaginalis based on restriction analysis of the ITS1 gene. In another study by Conrad et al. (2012) where the authors used multilocus sequence typing and microsatellite genotyping of T. vaginalis, a correlation between genotypes and metronidazole susceptibility patterns was observed using microsatellite genotyping. A future research direction for our research group would be to determine genotypes based on microsatellite genotyping and then attempt to link the data to patterns of susceptibility/resistance in order to determine correlations.
This study was limited in terms of due to the cross-sectional nature of this study, the women were not followed up in order to determine test of cure, and therefore, the clinical response could not be matched with in vitro susceptibility. Another limitation is that women were recruited from one hospital; however, the King Edward VIII Hospital is a tertiary hospital, and it hosts a wider population of Durban. Future collaboration studies are planned which will include T. vaginalis clinical isolates from other Districts in South Africa.
Conclusion
In this study, resistance to metronidazole was observed in clinical isolates of T. vaginalis. This was the first study to provide data on metronidazole susceptibility/resistance patterns in pregnant women from South Africa. Genotype G was shown to be the most dominant genotype in the study population. However, our study did not find a correlation between genotype harboured and metronidazole susceptibility/resistance patterns. Despite the lack of association, our study provides data on an area of research that is currently lacking in our setting.
References
Abdel-Magied AA, El-Kholya E-SI, Abou El-Khair SM, Abdelmegeed ES, Hamoudaa MM, Mohamed SA, El-Tantawy NL (2017) The genetic diversity of metronidazole susceptibility in Trichomonas vaginalis clinical isolates in an Egyptian population. Parasitol Res 116:3125–3130
Akbari Z, Matini M (2017) The study of trichomoniasis in pregnant women attending Hamadan City Health Centers in 2015. Avicenna. J Clin Microbiol Infect 4:e41533. https://doi.org/10.5812/ajcmi.41533
Alessio C, Nyirjesy P (2019) Management of resistant trichomoniasis. Curr Infect Dis Rep 21:1–7. https://doi.org/10.1007/s11908-019-0687-4
Bosserman EA, Helms DJ, Mosure DJ, Secor WE, Workowski KA (2011) Utility of antimicrobial susceptibility testing in Trichomonas vaginalis-infected women with clinical treatment failure. Sex Transm Dis 38:983–987. https://doi.org/10.1097/OLQ.0b013e318224db39
Bouchemal K, Bories C, Loiseaub PM (2017) Strategies for prevention and treatment of Trichomonas vaginalis infections. Clin Microbiol Rev 30:811–825
Bradic M, Warring SD, Tooley GE, Scheid P, Secor WE, Land KM, Huang PJ, Chen TW, Lee CC, Tang P, Sullivan SA, Carlton JM (2017) Genetic indicators of drug resistance in the highly repetitive genome of Trichomonas vaginalis. Genome Biol Evol 9:1658–1672. https://doi.org/10.1093/gbe/evx110
Conrad MD, Gorman AW, Schillinger JA, Fiori PL, Arroyo R, Malla N, Dubey ML, Gonzalez J, Blank S, Secor WE, Carlton JM (2012) Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis 6:e1573
Cornelius DC, Robinson DA, Muzny CA, Mena LA, Aanensen DM, Lushbaugh WB, Meadea JC (2012) Genetic characterization of Trichomonas vaginalis isolates by use of multilocus sequence typing. J Clin Microbiol 50:3293–3300. https://doi.org/10.1128/JCM.00643-12
Crucitti T, Abdellati S, Van Dyck E, Buvé A (2008) Molecular typing of the actin gene of Trichomonas vaginalis isolates by PCR–restriction fragment length polymorphism. Clin Microbiol Infect 14:844–852
Crucitti T, Jespers V, Mulenga C, Khondowe S, Vandepitte J, Buvé A (2010) Trichomonas vaginalis is highly prevalent in adolescent girls, pregnant women, and commercial sex workers in Ndola, Zambia. Sex Transm Dis 37:223–227
Diamond LS (1957) The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol 43:488–490
Espinosa N, Hernández R, Lopez-Griego L, Arroyo R, Lopez-Villasenor I (2001) Differences between coding and non-coding regions in the Trichomonas vaginalis genome: an actin gene as a locus model. Acta Trop 78:147–154
Gatti FA, Ceolan E, FSR G et al (2017) The prevalence of trichomoniasis and associated factors among women treated at a university hospital in southern Brazil. PLoS One 12:e0173604. https://doi.org/10.1371/journal.pone.0173604
Glehn MP, Sá LCEF, da Silva HDF, Machado ER (2017) Prevalence of Trichomonas vaginalis in women of reproductive age at a family health clinic. J Infect Dev Countries 11:269–276. https://doi.org/10.3855/jidc.8143
Johnston VJ, Mabey DC (2008) Global epidemiology and control of Trichomonas vaginalis. Curr Opin Infect Dis 21:56–64
Khalili B, Ghasemi-Dehkordi P, Pourshahbazi G, Yousofi-Darani H, Hashemzadeh-Chaleshtori M, Doosti A (2017) Genotyping of Trichomonas vaginalis isolates from women in Shahrekord city (Southwestern Iran). Genetika 49:1059–1070
Kirkcaldy RD, Augostini P, Asbel LE, Bernstein KT, Kerani RP, Mettenbrink CJ et al (2012) Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009-2010. Emerg Infect Dis 18:939–943. https://doi.org/10.3201/eid1806.111590
Kissinger P (2015a) Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep 17:1–9. https://doi.org/10.1007/s11908-015-0484-7
Kissinger P (2015b) Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis 15:307. https://doi.org/10.1186/s12879-015-1055-0
Kusdian G, Gould SB (2014) The biology of Trichomonas vaginalis in the light of urogenital tract infection. Mol Biochem Parasitol 198:92–99. https://doi.org/10.1016/j.molbiopara.2015.01.004
Mabaso N, Tinarwo P, Abbai N (2020) Lack of association between Mycoplasma hominis and Trichomonas vaginalis symbiosis in relation to metronidazole resistance. Parasitol Res 119:4197–4204. https://doi.org/10.1007/s00436-020-06930-x
Meade JC, Carlton JM (2013) Genetic diversity in Trichomonas vaginalis. Sex Transm Infect 89:444–448
Mielczarek E, Blaszkowska J (2015) Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection 44:447–458. https://doi.org/10.1007/s15010-015-0860-0
Momeni Z, Sadraei J, Kazemi B, Dalimi A (2015) Molecular typing of the actin gene of Trichomonas vaginalis isolates by PCR-RFLP in Iran. Exp Parasitol 159:259–263
Moodley D, Moodley P, Sebitloane M, Soowamber D, McNaughton-Reyes HL, Groves AK, Maman S (2015) High prevalence and incidence of asymptomatic sexually transmitted infections during pregnancy and postdelivery in KwaZulu Natal, South Africa. Sex Transm Dis 42:43–47. https://doi.org/10.1097/OLQ.0000000000000219
Morikawa E, Mudau M, Olivier D, de Vos L, Joseph Davey D, Price C, McIntyre JA, Peters RP, Klausner JD, Medina-Marino A (2018) Acceptability and feasibility of integrating point-of-care diagnostic testing of sexually transmitted infections into a South African Antenatal Care Program for HIV-infected pregnant women. Infect Dis Obstet Gynecol 2018:1–6. https://doi.org/10.1155/2018/3946862
Müller M, Lossick J, Gorrell T (1988) In vitro susceptibility of Trichomonas vaginalis to metronidazole and treatment outcome in vaginal trichomoniasis. Sex Transm Dis 15:17–24
Naidoo S, Wand H (2013) Prevalence and incidence of Trichomonas vaginalis infections in women participating in a clinical trial in Durban, South Africa. Sex Transm Infect 89:519–522. https://doi.org/10.1136/sextrans-2012-050984
Petrin D, Delgaty K, Bhatt R, Garber G (1998) Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev 11:300–317
Ramjee G, Abbai NS, Naidoo S (2015) Women and sexually transmitted infections in Africa. Open J Obstet Gynecol 5:385–399. https://doi.org/10.4236/ojog.2015.57056
Rezaeian M et al (2009) Prevalence of Trichomonas vaginalis using parasitological methods in Tehran. Iran J Parasitol 4:43–47
Rukasha I, Ehlers MM, Kock MM (2013) P5.099 Metronidazole antimicrobial drug resistance testing of Trichomonas vaginalis collected from women attending an anti-retroviral clinic, Pretoria, South Africa. Sex Transm Infect 89:A366.361–A366A366. https://doi.org/10.1136/sextrans-2013-051184.1143
Shahraki F, Fouladi B, Salimi-Khorashad A, Sepehri-Rad N, Dabirzadeh M (2020) Epidemiology and identification of actin gene of Trichomonas vaginalis genotypes in women of southeast of Iran using PCR-RFLP. Crescent J Medical Biol Sci 7:82–90
Shaio M, Lin P, Liu J (1997) Colorimetric one-tube nested PCR for detection of Trichomonas vaginalis in vaginal discharge. J Clin Microbiol 35:132–138
Sood S, Kapil A (2008) An update on Trichomonas vaginalis. Indian J Sex Transm Dis 29:7–14
Swygard H, Sena AC, Hobbs MM, Cohen MS (2004) Trichomoniasis: clinical manifestations, diagnosis and management. Sex Transm Infect 80:91–95. https://doi.org/10.1136/sti.2003.005124
Upcroft JA, Upcroft P (2001) Drug susceptibility testing of anaerobic protozoa. Antimicrob Agents Chemother 45:1810–1814. https://doi.org/10.1128/AAC.45.6.1810-1814.2001
Acknowledgements
The authors would like to thank all the women who participated in this study and the King Edward VIII Hospital Antenatal Clinic.
Availability of data and material
Not applicable
Code availability
Not applicable
Funding
This work was supported by the College of Health Science, University of KwaZulu-Natal, and the National Research Foundation (grant number: 112555).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Ethics approval for this study was obtained from the Biomedical Research Ethics Committee (BREC), University of KwaZulu-Natal (BE296/18).
Consent to participate
Written informed consent was obtained from the study participants.
Consent to publish
De-identified data has been obtained from our local Institutional Review Board (IRB).
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Nawal Hijjawi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mabaso, N., Abbai, N. Distribution of genotypes in relation to metronidazole susceptibility patterns in Trichomonas vaginalis isolated from South African pregnant women. Parasitol Res 120, 2233–2241 (2021). https://doi.org/10.1007/s00436-021-07177-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-021-07177-w