Abstract
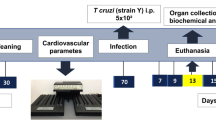
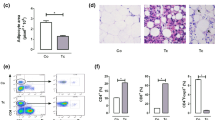
The underlying pathogenic mechanisms of cardiomyopathy in Chagas disease are still unsolved. In order to better clarify the role of fat on the evolution of cardiomyopathy, the present study employed three murine models of chronic Trypanosoma cruzi infection: (1) aP2-RIDα/β transgenic mice (RID mice; an adipose tissue model which express a gain-of-function potent anti-inflammatory activity), (2) allograft inflammatory factor-1 knockout mice (Aif1−/−), and (3) a Swiss outbred mice. RID mice and non-transgenic mice (wild type, WT) were infected with blood trypomastigotes of Brazil strain. During the acute stage of infection, RID mice had lower parasitemia, lower heart inflammation, and a decrease in the relative distribution of parasite load from cardiac muscle tissue toward epididymal fat. Nevertheless, comparable profiles of myocardial inflammatory infiltrates and relative distribution of parasite load were observed among RID and WT at the chronic stage of infection. Aif1−/− and Aif1+/+ mice were infected with bloodstream trypomastigotes of Tulahuen strain and fed with high-fat diet (HFD) or regular diet (RD). Interestingly, Aif1+/+ HFD infected mice showed the highest mortality. Swiss mice infected with blood trypomastigotes of Berenice-78 strain on a HFD had higher levels of TNFα and more inflammation in their heart tissue than infected mice fed a RD. These various murine models implicate adipocytes in the pathogenesis of chronic Chagas disease and suggest that HFD can lead to a significant increase in the severity of parasite-induced chronic cardiac damage. Furthermore, these data implicate adipocyte TLR4-, TNFα-, and IL-1β-mediated signaling in pro-inflammatory pathways and Aif-1 gene expression in the development of chronic Chagas disease.














Similar content being viewed by others
Change history
20 April 2020
The authors regret that Philipp E Scherer’s name was spelt incorrectly in the author list. The name of the author is now corrected above.
References
Arslan N, Erdur B, Aydin A (2010) Hormones and cytokines in childhood obesity. Indian Pediatr 47:829–839
Bahia MT, Diniz Lde F, Mosqueira VC (2014) Therapeutical approaches under investigation for treatment of Chagas disease. Expert Opin Investig Drugs 23(9):1225–1237
Benvenuti LA, Rogério A, Freitas HFG et al (2008) Chronic American trypanosomiasis: parasite persistence in endomyocardial biopsies is associated with high-grade myocarditis. Ann Trop Med Parasitol 102(6):481–487
Bern C (2015) Chagas’ disease. N Engl J Med 373:456–466
Brener Z (1962) Therapeutic activity and criterion of cure in mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop 4:389–396
Briceno-Leon R, Mendez Galvan J (2007) The social determinants of Chagas disease and the transformations of Latin America. Mem Inst Oswaldo Cruz 102(1):109–121
Bustamante JM, Craft JM, Crowe BD, Ketchie SA, Tarleton RL (2013) New, combined, and reduced dosing treatment protocols cure Trypanosoma cruzi infection in mice. J Infect Dis 209(1):150–162
Cabalén ME, Cabral MF, Sanmarco LM, Andrada MC, Onofrio LI, Ponce NE, Aoki MP, Gea S, Cano RC (2016) Chronic Trypanosoma cruzi infection potentiates adipose tissue macrophage polarization toward an anti-inflammatory M2 phenotype and contributes to diabetes progression in a diet-induced obesity model. Oncotarget 7(12):13400–13415
Caldas IS, Talvani A, Caldas S, Carneiro CM, de Lana M, da Matta Guedes PM, Bahia MT (2008) Benznidazole therapy during acute phase of Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol Res 103:413–421
Caldas IS, Guedes PMM, Santos FM et al (2013) Myocardial scars correlate with eletrocardiographic changes in chronic Trypanosoma cruzi infection for dogs treated with benznidazole. Tropical Med Int Health 18(1):75–84
Casimiro I, Chinnasamy P, Sibinga NES (2013) Genetic inactivation of the allograft inflammatory factor-1 locus. Genesis 51(10):1–13
Castro-Sesquen YE, Gilman RH, Yauri V, Angulo N, Verastegui M, Velásquez DE, Sterling CR, Martin D, Bern C (2011) Cavia porcellus as a model for experimental infection by Trypanosoma cruzi. Am J Pathol 179(1):281–288
Chagas C (1909) Nova tripanozomiaze humana. Estudos sobre a morfologia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp. Agente etiológico de uma nova entidade mórbida para o homem. Mem Inst Oswaldo Cruz 1:159–218
Chapadeiro E, Beraldo PS, Jesus PC et al (1988) Cardiac lesions in Wistar rats inoculated with various strains of Trypanosoma cruzi. [Article in Portuguese.]. Rev Soc Bras Med Trop 21:95–103
Chin YR, Horwitz MS (2005) Mechanism for removal of tumor necrosis factor receptor 1 from the cell surface by the adenovirus RIDα/β complex. J Virol 79(21):13606–13617
Coura JR (2007) Chagas disease: what is known and what is needed—a background article. Mem Inst Oswaldo Cruz 102(1):113–122
Coura JR, Viñas PA (2010) Chagas disease: a new worldwide challenge. Nature 465(7301):S6–S7
D’Ávila DA, Guedes PM, Castro AM et al (2009) Immunological imbalance between IFN-g and IL-10 levels in the sera of patients with the cardiac form of Chagas disease. Mem Inst Oswaldo Cruz 104:100–105
del Puerto F, Nishizawa JE, Kikuchi M et al (2012) Protective human leucocyte antigen haplotype, HLA-DRB1*01-B*14, against chronic Chagas disease in Bolivia. Neglected Tropical Diseases 6(3):e1587
Estadella D, Oyama LM, Dâmaso AR, Ribeiro EB, Oller do Nascimento CM (2004) Effect of palatable hyperlipidic diet on lipid metabolism of sedentary and exercised rats. Nutrition 20:218–224
Fernandes JL, Soeiro A, Ferreira CB et al (2006) Acute coronary syndromes and inflammation. Rev Soc Cardiol 3:178–187
Garcia S, Ramos CO, Senra JFV et al (2005) Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob Agents Chemother 49(4):1521–1528
Guedes PM, Fietto JLR, Lana M, Bahia MT (2006) Advances in Chagas disease chemotherapy. Anti Cancer Agents Med Chem 5:175–186
Guedes PMM, Veloso VM, Afonso LCC et al (2009) Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-g, TNF-a, and low IL-10 production during the acute infection phase. Vet Immunol Immunophatol 130:43–52
Gutierrez FR, Guedes PM, Gazinelli RT et al (2009) The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol 31:673–685
Kayama H, Takeda K (2010) The innate immune response to Trypanosoma cruzi infection. Microbes Infect 12:511–517
Lula JF, Rocha MO, Nunes MC et al (2009) Plasma concentrations of tumor necrosis factor-a, tumor necrosis factor-related apoptosis-inducing ligand, and FasLigand/CD95L in patients with Chagas cardiomyopathy correlate with left ventricular dysfunction. Eur J Heart Fail 11:825–831
Macedo AM, Pena SDJ (1998) Genetic variability of Trypanosoma cruzi: implications for the pathogenesis of Chagas disease. Parasitol Today 14(3):119–124
Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV (2007) Pathogenesis of chronic Chagas heart disease. Circulation 115:1109–1123
Moolani Y, Bukhman G, Hotez PJ (2012) Neglected tropical diseases as hidden causes of cardiovascular disease. PLoS Negl Trop Dis 6(6):e1499
Morillo CA, Marin-Neto JA, Avezum A et al (2015) Randomized trial of benznidazole for chronic Chagas´ cardiomyopathy. N Engl J Med 373(14):1925–1306
Morris SA, Weiss LM, Factor S, Bilezikian JP, Tanowitz H, Wittner M (1989) Verapamil ameliorates clinical, pathologic and biochemical manifestations of experimental chagasic cardiomyopathy in mice. J Am Coll Cardiol 14(3):782–789
Nagajyothi F, Desruisseaux MS, Machado FS, Upadhya R, Zhao D, Schwartz GJ, Teixeira MM, Albanese C, Lisanti MP, Chua SC Jr, Weiss LM, Scherer PE, Tanowitz HB (2012) Response of adipose tissue to early infection with Trypanosoma cruzi (Brazil strain). J Infect Dis 205(5):830–840
Nagajyothi F, Weiss LM, Zhao D, Koba W, Jelicks LA, Cui MH, Factor SM, Scherer PE, Tanowitz HB (2014) High fat diet modulates Trypanosoma cruzi infection associated myocarditis. PLoS Negl Trop Dis 8(10):e3118
Rassi A Jr, Rassi A, Rassi SG (2007) Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation 115:1101–1108
Samudio M, Montenegro-James S, Cabral M, Martinez J, Rojas de Arias A, James MA (1998) Cytokine responses in Trypanosoma cruzi-infected children in Paraguay. Am J Trop Med Hyg 58:119–121
Sanches TLM, Cunha LD, Silva GK, Guedes PM, Silva JS, Zamboni DS (2014) The use of a heterogeneously controlled mouse population reveals a significant correlation of acute phase parasitemia with mortality in Chagas disease. PLoS One 9(3):e91640
Santos FM, Lima WG, Gravel AS, Martins TA, Talvani A, Torres RM, Bahia MT (2012) Cardiomyopathy prognosis after benznidazole treatment in chronic canine Chagas’ disease. J Antimicrob Chemother 67:1987–1995
Santos FM, Mazzeti AL, Caldas S et al (2016) Chagas cardiomyopathy: the potential effect of benznidazole treatment on diastolic dysfunction and cardiac damage in dogs chronically infected with Trypanosoma cruzi. Acta Trop 161:44–54
Schmunis GA (2007) Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz 102(1):75–85
Schmunis GA, Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115(1–2):14–21
Tanowitz HB, Machado FS, Jelicks LA, Shirani J, de Carvalho AC, Spray DC, Factor SM, Kirchhoff LV, Weiss LM (2009) Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease). Prog Cardiovasc Dis 51:524–539
Tanowitz HB, Scherer PE, Mota MM, Figueiredo LM (2017) Adipose tissue: a safe haven for parasites? Trends Parasitol 33(4):276–284
Urbina JÁ (2009) Ergosterol biosynthesis and drug development for Chagas disease. Mem Inst Oswaldo Cruz 104(1):311–318
Vago AR, Andrade LO, Leite AA, d'Avila Reis D, Macedo AM, Adad SJ, Tostes S Jr, Moreira MC, Filho GB, Pena SD (2000) Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol 156:1805–1809
Winkler G, Kiss S, Keszthelyi L et al (2003) Expression of tumor necrosis factor (TNF-alpha) protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C- peptide level. Eur J Endrocrinol 149(2):129–135
Funding
This work was supported by the Brazilian institutions Fundação de Amparo à Pesquisa e Inovação do Espírito Santo (FAPES, grant term number 565/2015), Federal University of Espírito Santo (UFES), and by a grant from the United States of America National Institute of Allergy and Infectious Diseases AI124000.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures and experimental protocols concerning experiments with transgenic aP2-RID α/β mouse model and Aif-1 target inactivated mouse model were approved by the Institutional Animal Care and Use Committees (IACUC) of Albert Einstein College of Medicine (No. 20151206) and conducted in accordance with the guidelines of the National Research Council (Guide for Care and Use of Laboratory Animals: Eight Edition, Washington, DC: The National Academic Press, 2011).
All procedures or experimental protocols conducted in the Swiss mouse model were performed according to CONCEA (National Council for Control of Animal Experimentation) and behavior instructions for the use of animals in research from Brazil. The experiment with Swiss mouse model was also previously approved by the Ethics Committee on the Use of Animals of the Federal University of Espírito Santo (protocol number 43/2015).
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zaki, P., Domingues, E.L., Amjad, F.M. et al. The role of fat on cardiomyopathy outcome in mouse models of chronic Trypanosoma cruzi infection. Parasitol Res 119, 1829–1843 (2020). https://doi.org/10.1007/s00436-020-06645-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06645-z