Abstract
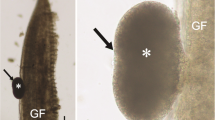
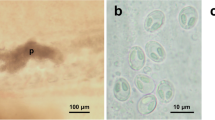
This paper provides morphological and phylogenetic analyses of two new myxobolid species found infecting Piaractus brachypomus from the Amazon basin. The fish were caught in the Tapajós River, in the municipality of Santarém, in the state of Pará, Brazil. The plasmodial development of Henneguya brachypomus n. sp. occurred in the gill lamellae while Myxobolus pirapitingae n. sp. developed in the pyloric cecum. Morphological analyses did not identify inflammatory infiltrate for either species, but H. brachypomus n. sp. induced stretching of the epithelium, compression of the adjacent tissues, and displacement and deformation of the neighboring lamellae. The mature myxospores of H. brachypomus n. sp. were ellipsoid, with a length of 11.7–13.8 μm, a width of 4.0–4.6 μm, and a thickness of 3.5˗4.3 μm. The polar capsules were elongated, with a length of 5.6–7.3 μm and a width of 1.3–2.0 μm, and each contained a polar filament with 8–9 coils. The caudal process was 40.5–48.1 μm long and the total length of the myxospore was 52.4–61.6 μm. Myxobolus pirapitingae n. sp. exhibited rounded mature myxospores measuring 10.0–11.1 μm in length, 7.0–7.6 μm in width, and 5.4–6.3 μm in thickness. The polar capsules were of equal size and occupied less than half the myxospore, measuring 3.5–4.0 μm in length and 2.0–2.6 μm in width, with each containing a polar filament with 6–7 coils. Phylogenetic analysis based on partial small subunit ribosomal DNA (ssrDNA) sequences showed that H. brachypomus n. sp. clustered as a sister species of Henneguya piaractus, while M. pirapitingae n. sp. was grouped in a sub-clade together with Myxobolus matosi and Myxobolus colossomatis.








Similar content being viewed by others
References
Adriano EA, Oliveira OMP (2019) Myxosporea in Catálogo Taxonômico da Fauna do Brasil. PNUD. Disponível em: <http://fauna.jbrj.gov.br/fauna/faunadobrasil/152799. Accessed 9 Jan 2019
Adriano EA, Arana S, Cordeiro NS (2005) Histology, ultrastructure and prevalence of Henneguya piaractus (Myxosporea) infecting the gills of Piaractus mesopotamicus (Characidae) cultivated in Brazil. Dis Aquat Org 64:229–235
Adriano EA, Arana S, Cordeiro NS (2006) Myxobolus cuneus n. sp. (Myxosporea) infecting the connective tissue of Piaractus mesopotamicus (Pisces: Characidae) in Brazil: histopathology and ultrastructure. Parasite 13:137–142
Adriano EA, Arana S, Alves AL, Silva MR, Ceccarelli PS, Henrique-Silva F, Maia AA (2009) Myxobolus cordeiroi n. sp., a parasite of Zungaro jahu (Siluriformes: Pimelodiade) from Brazilian Pantanal: morphology, phylogeny and histopathology. Vet Parasitol 162:221–229. https://doi.org/10.1016/j.vetpar.2009.03.030
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLASTn and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3392
Azevedo C, Matos E (2003) Fine structure of Henneguya pilosa sp. n. (Myxozoa: Myxosporea), parasite of Serrasalmus altuvei (Characidae), in Brazil. Folia Parasitol 50:37–42
Azevedo C, Clemente SCS, Casal G, Matos P, Alves A, Al-Quraishy S, Matos E (2012) Myxobolus myleus n. sp. infecting the bile of the Amazonian freshwater fish Myleus rubripinnis (Teleostei: Serrasalmidae): morphology and pathology. Syst Parasitol 82:241–247
Barassa B, Adriano EA, Arana S, Cordeiro NS (2003) Henneguya curvata sp. n. (Myxosporea, Myxobolidae) parasitizing the gills of Serrasalmus spilopleura (Characidae: Serrasalminae) a South American freshwater fish. Folia Parasitol 50:151–153
Barta JR, Martin DS, Liberator PA, Dashkevicz M, Anderson JW, Feighner SD, Elbrecht A, Perkinsbarrow A, Jenkins MC, Danforth HD, Ruff MMD, Profousjuchelka H (1997) Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J Parasitol 83:262–271
Canning EU, Okamura B (2004) Biodiversity and evolution of the Myxozoa. Adv Parasitol 56:43–131
Capodifoglio KRH, Adriano EA, Silva MRM, Maia AAM (2015) Supplementary data of Henneguya leporinicola (Myxozoa, Myxosporea) a parasite of Leporinus macrocephalus from fish farms in the state of São Paulo, Brazil. Acta Parasitol 60(3):451–458
Capodifoglio KRH, Adriano EA, Silva MRM, Maia AAM (2019) The resolution of the taxonomic dilemma of Myxobolus colossomatis and description of two novel myxosporeans species of Colossoma macropomum from Amazon basin. Acta Trop 191:17–23
Casal G, Matos E, Azevedo C (2002) Ultrastructural data on the spore of Myxobolus maculatus n. sp. (phylum Myxozoa), parasite from the Amazonian fish Metynnis maculatus (Teleostei). Dis Aquat Org 51:107–112
Casal G, Matos E, Azevedo C (2006) A new myxozoan parasite from the Amazonian fish Metynnis argenteus (Teleostei, Characidae): light and electron microscope observations. J Parasitol 92:817–821
Eiras JC, Adriano EA (2012) A checklist of new species of Henneguya Thélohan, 1892 (Myxozoa: Myxosporea, Myxobolidae) described between 2002 and 2012. Syst Parasitol 83:95–104
Eiras JC, Molnár K, Lu YS (2005) Synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea: MyxoBolidae). Syst Parasitol 61(1):1–46
Eiras JC, Zhang J, Molnár K (2014) Synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea: Myxobolidae) described between 2005 and 2013. Syst Parasitol 88:11–36
El-Mansy A, Bashtar AR (2002) Histopathological and ultrastructural studies of Henneguya suprabranchiae Landsberg, 1987 (Myxosporea: Myxobolidae) parasitizing the suprabranchial organ of the freshwater catfish Clarias gariepinus Burchell, 1822 in Egypt. Parasitol Res 88(7):617–626
FAO (2015) The Food and Agriculture Organization. http://www.fao.org/nr/water/aquastat/basins/amazon/print1.stm. Accessed 19 August 2019
Flores Nava A (2007) Aquaculture seed resources in Latin America: a regional synthesis. In: Bondad-Reantaso MG (ed) Assessment of Freshwater Fish Seed Resources for Sustainable Aquaculture. FAO Fisheries Technical Paper, No. 501, FAO, Rome, pp 91–102
Fresneda A, Lenis G, Agudelo E, Olivera Ángel M (2004) Espermiación inducida y crioconservación de semen de cachama blanca (Piaractus brachypomus). Rev Colomb Cienc Pec 17:46–52
Friesen VL, Congdon BC, Kidd MG, Birt TP (1999) Polymerase chain reaction (PCR) primers for the amplification of five nuclear introns in vertebrates. Mol Ecol 8
Froese R, Pauly D (Eds.) (2017) FishBase. World Wide Web electronic publication, www.fishbase.org (Accessed April 2018)
Goulding M, Smith NJH, Mahar DJ (1996) Floods of fortune: ecology and economy along the Amazon. Columbia University Press, New York
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hallet SL, Diamant A (2001) Ultrastructure and small- subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland, Australia. Dis Aquat Org 46:197–212
Honglang H (2007) Freshwater fish seed resources in China. In: Bondad-Reantaso MG (ed) Assessment of Freshwater Fish Seed Resources for Sustainable Aquaculture, FAO Fisheries Technical Paper No. 501. FAO, Rome, pp 185–199
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0. Mol Biol Evol 33(7):1870–1874
Lin Y, Gao Z, Zhan A (2015) Introduction and use of non-native species for aquaculture in China status, risk and management solutions. Rev Aquac 7:28–58. https://doi.org/10.1111/raq.12052
Lom J, Arthur JR (1989) A guideline for the preparation of species description in Myxosporea. J Fish Dis 12:151–156
Madden T (2002) The BLAST sequence analysis tool. In: McEntyre J, Ostell J (eds) The NCBI Handbook [Internet]. National Center for Biotechnology Information (US), Bethesda (MD) https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome. Accessed on Nov. 19th 2018
Martins ML, Souza VN (1997) Henneguya piaractus n. sp. (Myxozoa: Myxobolidae), a gill parasite of Piaractus mesopotamicus Holmberg, 1887 (Osteichthyes: Characidae), in Brazil. Rev Bras Biol 57:239–245
Martins ML, Souza VN, Moraes ER, Moraes JRE, Costa AJ, Rocha UE (1997) Pathology and behavioural effects associated with Henneguya sp. (Myxozoa: Myxobolidae) infections of captive pacu Piaractus mesopotamicus in Brazil. J World Aquacult Soc 28:297–300
Milanin T, Maia AAM, Silva MRM, Carriero MM, Adriano EA (2015) Molecular phylogeny and ultrastructure of Myxobolus cf. cuneus, a parasite of patinga hybrid and Henneguya pseudoplatystoma, a parasite of pintado hybrid. Acta Parasitol 60(3):442–450
Molnár K, Békési L (1993) Description of a new Myxobolus species, M. colossomatis n. sp. from the teleost Colossoma macropomum of the Amazon River basin. J Appl Ichthyol 9:57–63
Molnár K, Eszterbauer E, Székely C, Dán A, Harrach B (2002) Morphological and molecular biological studies on intramuscular Myxobolus spp. of cyprinid fish. J Fish Dis 25:643–652
MPA–Ministério da Pesca e Aquicultura (2012) Boletim Estatístico daPesca e Aquicultura. Brasília
Müller MI, Adriano EA, Ceccarelli PS, Silva MRM, Ueta MT (2013) Prevalence, intensity, and phylogenetic analysis of Henneguya piaractus and Myxobolus cf. colossomatis from farmed Piaractus mesopotamicus in Brazil. Dis Aquat Org 107(2):129–139
Naldoni J, Maia AAM, Correa LL, Silva MRM, Adriano EA (2018) New myxosporeans parasitizing Phractocephalus hemioliopterus from Brazil: morphology, ultrastructure and SSU-rDNA sequencing. Dis Aquat Org 128:37–49
Okamura B, Gruhl A, Bartholomew JL (2015) An introduction of myxozoan evolution, ecology and development. In: Okamura B, Gruhl A, Bartholomew JL (eds) Myxozoan evolution, ecology and development. Springer, Cham, pp 1–22
Okamura B, Hartigan A, Naldoni J (2018) Extensive uncharted biodiversity: the parasite dimension. Integr Comp Biol:1–14. https://doi.org/10.1093/icb/icy039
Ovcharenko M, Dezfulli BS, Castaldelli G, Lanzoni M, Giaria L (2017) Histological and ultrastructural study of Myxobolus mugchelo (Parenzan, 1966) with initial histopathology survey of the Liza ramada host intestine. Parasitol Res 116:1713–1721
Penido JCN (1927) Quelques nouvelles myxosporidies parasites des poissons d’eau douce du Brésil. C R Séances Socied Biol 97:850–852
Pinto C (1928) Myxobolus noguchi, M. stokesi e Henneguya iheringi, espécies novas de Myxosporideos de peixes de água doce do Brasil. Bol Biol 12:41–43 1928
Rambaut A (2008) FigTree v1.1.1: tree figure drawing tool. [http://tree.bio.ed.ac.uk/software/figtree/] (Accessed: 18 April 2017)
Rocha E, Matos E, Azevedo C (1992) Henneguya amazônica n. sp. (Myxozoa, Myxobolidae), parasitising the gills of Crenicichla lepidota Heckel, 1840 (Teleostei, Cichlidae) from Amazon river. Eur J Protistol 28:173–278
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):11572–11574
Santos GM, Santos ACM (2005) Sustentabilidade da pesca na Amazônia. Estudos Avançados 19:165–182
Sitja-Bobadilla A (2008) Fish immune response to Myxozoan parasites. Parasite 15(3):420–425
Sobrinho A, Silva A, Melo F (1984) Resultados de um experimento de policultivo da pirapitinga Collossoma brachypomus Cuvier, 1818, com o hibrido de tilápias (Oreochromishomorum niloticus). Bol Téc DNOCS 42(1):91–115
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A (2018) Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 4(1). https://doi.org/10.1093/ve/vey016
Thompson JD, Higgins DG, Gilson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Val AL, Honczaryk A (1995) Criando peixes na Amazônia, 19th edn. INPA, Manaus
Walliker D (1969) Myxosporidea of some Brazilian fresh water fishes. J Parasitol 55:942–948
Funding
The present study was supported by grants nos. 2013/21374-6 and 2016/22047-7 of the São Paulo Research Foundation (FAPESP) to E. A. Adriano. K. R. H. Capodifoglio was supported by scholarship no. 2015/07713-8 of the São Paulo Research Foundation (FAPESP). C. M. Meira was supported by scholarship no. 2016/19149-2 of the São Paulo Research Foundation (FAPESP). J. Naldoni was supported by scholarship no. 2014/22700-7 of the São Paulo Research Foundation (FAPESP). The study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (the Coordination for the Improvement of Higher Education Personnel) - Brasil (CAPES)—Finance Code 001. E. A. Adriano received a research productivity grant from the Brazilian Fostering Agency CNPq (grant no. 301886/2016-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Section Editor: Christopher Whipps
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Capodifoglio, K.R.H., Adriano, E.A., Naldoni, J. et al. Novel myxosporean species parasitizing an economically important fish from the Amazon basin. Parasitol Res 119, 1209–1220 (2020). https://doi.org/10.1007/s00436-020-06641-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06641-3