Abstract
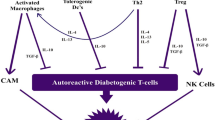
Infection with helminth parasites or the administration of their antigens can prevent or attenuate autoimmune diseases. To date, the specific molecules that prime the amelioration are only limited. In this study, recombinant Schistosoma japonicum cystatin (rSjcystatin) and fructose-1,6-bisphosphate aldolase (rSjFBPA) were administered to female NOD mice via intraperitoneal (i.p.) injection to characterize the immunological response by the recombinant proteins. We have shown that the administration of rSjcystatin or rSjFBPA significantly reduced the diabetes incidence and ameliorated the severity of type 1 diabetes mellitus (T1DM). Disease attenuation was associated with suppressed interferon-gamma (IFN-γ) production in autoreactive T cells and with a switch to the production of Th2 cytokines. Following rSjcystatin or rSjFBPA injection, regulatory T cells (Tregs) were remarkably increased, which was accompanied by increased expression of interleukin-10 (IL-10) and transforming growth factor beta (TGF-β). Our study suggests that helminth-derived proteins may be useful in strategies to limit pathology by promoting the Th2 response and upregulating Tregs during the inflammatory tissue-damage process in T1DM.







Similar content being viewed by others
Abbreviations
- rSjcystatin:
-
recombinant Schistosoma japonicum cystatin
- rSjFBPA:
-
recombinant Schisotosoma japonicum fructose-1,6-bisphosphate aldolase
- T1DM:
-
type 1 diabetes mellitus
- CY:
-
cyclophosphamide
- NOD:
-
nonobese diabetic
- Tregs:
-
regulatory T cells
- ELISA:
-
enzyme-linked immunosorbent assay
- SEA:
-
soluble egg antigen
- SWA:
-
soluble worm antigen
- IBD:
-
inflammatory bowel disease
References
Ablamunits V, Quintana F, Reshef T, Elias D, Cohen IR (1999) Acceleration of autoimmune diabetes by cyclophosphamide is associated with an enhanced IFN-gamma secretion pathway. J Autoimmun 13:383–392
Bach JF (2018) The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18:105–120
Berbudi A, Ajendra J, Wardani AP, Hoerauf A, Hubner MP (2016) Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes Metab Res Rev 32:238–250
Brode S, Raine T, Zaccone P, Cooke A (2006) Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 177:6603–6612
Chiang JL, Kirkman MS, Laffel LM, Peters AL (2014) Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 37:2034–2054
Driver JP, Serreze DV, Chen YG (2011) Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol 33:67–87
Du L, Tang H, Ma Z, Xu J, Gao W, Chen J, Gan W, Zhang Z, Yu X, Zhou X, Hu X (2011) The protective effect of the recombinant 53-kDa protein of Trichinella spiralis on experimental colitis in mice. Dig Dis Sci 56:2810–2817
Espinoza-Jimenez A, De Haro R, Terrazas LI (2017) Taenia crassiceps antigens control experimental type 1 diabetes by inducing alternatively activated macrophages. Mediat Inflamm 2017:8074329
Everts B, Hussaarts L, Driessen NN, Meevissen MH, Schramm G, van der Ham AJ, van der Hoeven B, Scholzen T, Burgdorf S, Mohrs M, Pearce EJ, Hokke CH, Haas H, Smits HH, Yazdanbakhsh M (2012) Schistosome-derived omega-1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med 209(1753-1767):S1
Han D, Leyva CA, Matheson D, Mineo D, Messinger S, Blomberg BB, Hernandez A, Meneghini LF, Allende G, Skyler JS, Alejandro R, Pugliese A, Kenyon NS (2011) Immune profiling by multiple gene expression analysis in patients at-risk and with type 1 diabetes. Clin Immunol 139:290–301
He B, Cai G, Ni Y, Li Y, Zong H, He L (2011) Characterization and expression of a novel cystatin gene from Schistosoma japonicum. Mol Cell Probes 25:186–193
Hubner MP, Shi Y, Torrero MN, Mueller E, Larson D, Soloviova K, Gondorf F, Hoerauf A, Killoran KE, Stocker JT, Davies SJ, Tarbell KV, Mitre E (2012) Helminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-beta. J Immunol 188:559–568
Jang SW, Cho MK, Park MK, Kang SA, Na BK, Ahn SC, Kim DH, Yu HS (2011) Parasitic helminth cystatin inhibits DSS-induced intestinal inflammation via IL-10(+)F4/80(+) macrophage recruitment. Korean J Parasitol 49:245–254
Johnson MC, Wang B, Tisch R (2011) Genetic vaccination for re-establishing T-cell tolerance in type 1 diabetes. Hum Vaccin 7:27–36
Joseph J, Bittner S, Kaiser FM, Wiendl H, Kissler S (2012) IL-17 silencing does not protect nonobese diabetic mice from autoimmune diabetes. J Immunol 188:216–221
Khan AR, Fallon PG (2013) Helminth therapies: translating the unknown unknowns to known knowns. Int J Parasitol 43:293–299
Kordis D, Turk V (2009) Phylogenomic analysis of the cystatin superfamily in eukaryotes and prokaryotes. BMC Evol Biol 9:266
Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D (2011) Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A 108:11548–11553
Lambrecht BN, Hammad H (2017) The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol 18:1076–1083
Lau K, Benitez P, Ardissone A, Wilson TD, Collins EL, Lorca G, Li N, Sankar D, Wasserfall C, Neu J, Atkinson MA, Shatz D, Triplett EW, Larkin J 3rd. (2011) Inhibition of type 1 diabetes correlated to a Lactobacillus johnsonii N6.2-mediated Th17 bias. J Immunol 186:3538–3546
Lipman TH, Ratcliffe SJ, Cooper R, Levitt Katz LE (2013) Population-based survey of the prevalence of type 1 and type 2 diabetes in school children in Philadelphia. J Diabetes 5:456–461
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Lund ME, O'Brien BA, Hutchinson AT, Robinson MW, Simpson AM, Dalton JP, Donnelly S (2014) Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS One 9:e86289
Marques HH, Zouain CS, Torres CB, Oliveira JS, Alves JB, Goes AM (2008) Protective effect and granuloma down-modulation promoted by RP44 antigen a fructose 1,6 bisphosphate aldolase of Schistosoma mansoni. Immunobiology 213:437–446
Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C (2009) Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol 39:216–224
McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmuller U, Baron U, Olek S, Bluestone JA, Brusko TM (2011) Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 186:3918–3926
McSorley HJ, Maizels RM (2012) Helminth infections and host immune regulation. Clin Microbiol Rev 25:585–608
Meevissen MH, Wuhrer M, Doenhoff MJ, Schramm G, Haas H, Deelder AM, Hokke CH (2010) Structural characterization of glycans on omega-1, a major Schistosoma mansoni egg glycoprotein that drives Th2 responses. J Proteome Res 9:2630–2642
Mishra PK, Patel N, Wu W, Bleich D, Gause WC (2013) Prevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type response. Mucosal Immunol 6:297–308
Mutapi F, Bourke C, Harcus Y, Midzi N, Mduluza T, Turner CM, Burchmore R, Maizels RM (2011) Differential recognition patterns of Schistosoma haematobium adult worm antigens by the human antibodies IgA, IgE, IgG1 and IgG4. Parasite Immunol 33:181–192
Quintana FJ, Carmi P, Cohen IR (2002) DNA vaccination with heat shock protein 60 inhibits cyclophosphamide-accelerated diabetes. J Immunol 169:6030–6035
Roep BO (2003) The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia 46:305–321
Saber M, Diab T, Hammam O, Karim A, Medhat A, Khela M, El-Dabaa E (2013) Protective and anti-pathology effects of Sm fructose-1,6-bisphosphate aldolase-based DNA vaccine against schistosoma mansoni by changing route of injection. Korean J Parasitol 51:155–163
Saunders KA, Raine T, Cooke A, Lawrence CE (2007) Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun 75:397–407
Sun Z, Shen B, Wu H, Zhou X, Wang Q, Xiao J, Zhang Y (2015) The secreted fructose 1,6-bisphosphate aldolase as a broad spectrum vaccine candidate against pathogenic bacteria in aquaculture. Fish Shellfish Immunol 46:638–647
Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA (2004) In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199:1455–1465
Tang CL, Zou JN, Zhang RH, Liu ZM, Mao CL (2019) Helminths protect against type 1 diabetes: effects and mechanisms. Parasitol Res 118:1087–1094
Teodorowicz M, Perdijk O, Verhoek I, Govers C, Savelkoul HF, Tang Y, Wichers H, Broersen K (2017) Optimized Triton X-114 assisted lipopolysaccharide (LPS) removal method reveals the immunomodulatory effect of food proteins. PLoS One 12:e0173778
Tong Z, Liu W, Yan H, Dong C (2015) Interleukin-17A deficiency ameliorates streptozotocin-induced diabetes. Immunology 146:339–346
Wang S, Xie Y, Yang X, Wang X, Yan K, Zhong Z, Wang X, Xu Y, Zhang Y, Liu F, Shen J (2016) Therapeutic potential of recombinant cystatin from Schistosoma japonicum in TNBS-induced experimental colitis of mice. Parasit Vectors 9:6
Yang X, Liu J, Yue Y, Chen W, Song M, Zhan X, Wu Z (2014) Cloning, expression and characterisation of a type II cystatin from Schistosoma japonicum, which could regulate macrophage activation. Parasitol Res 113:3985–3992
Yasunami R, Bach JF (1988) Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur J Immunol 18:481–484
Yi Z, Li L, Garland A, He Q, Wang H, Katz JD, Tisch R, Wang B (2012) IFN-gamma receptor deficiency prevents diabetes induction by diabetogenic CD4+, but not CD8+, T cells. Eur J Immunol 42:2010–2018
Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A (2003) Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol 33:1439–1449
Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A (2009) Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol 39:1098–1107
Zaccone P, Burton OT, Gibbs SE, Miller N, Jones FM, Schramm G, Haas H, Doenhoff MJ, Dunne DW, Cooke A (2011) The S. mansoni glycoprotein omega-1 induces Foxp3 expression in NOD mouse CD4(+) T cells. Eur J Immunol 41:2709–2718
Zhong ZR, Zhou HB, Li XY, Luo QL, Song XR, Wang W, Wen HQ, Yu L, Wei W, Shen JL (2010) Serological proteome-oriented screening and application of antigens for the diagnosis of Schistosomiasis japonica. Acta Trop 116:1–8
Acknowledgments
We thank Dr. Li He at Wuhan University School of Medicine for providing rSjcystatin plasmid.
Funding
The work was funded by the Natural Science Foundation of Anhui Province (No 1308085MH124) and the Natural Science Research of Educational Department Anhui Province (No. KJ2015ZD31).
Author information
Authors and Affiliations
Contributions
KY and XYX were responsible for all experiments and drafted the manuscript. BW, HBZ, and QLL helped in the analysis and interpretation of tests. ZRZ and JLS were responsible for design of the study and reviewed the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Bengbu Medical College. And the consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Section Editor: Xing-Quan Zhu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key findings
Administration of rSjcystatin or rSjFBPA could reduce the incidence and ameliorated the severity of T1DM. Disease attenuation was associated with suppressed IFN-γ production and a shift of Th1 to Th2. And Tregs were remarkably increased, which was accompanied by increased expression of IL-10 and TGF-β.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Yan, K., Wang, B., Zhou, H. et al. Amelioration of type 1 diabetes by recombinant fructose-1,6-bisphosphate aldolase and cystatin derived from Schistosoma japonicum in a murine model. Parasitol Res 119, 203–214 (2020). https://doi.org/10.1007/s00436-019-06511-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06511-7