Abstract
Intestinal opportunistic infections are often caused by unicellular parasites. Individuals with decreased immunity are particularly susceptible to infection by said microorganisms, and when they are infected, diarrhea can be the main clinical manifestation. However, intestinal parasites have rarely been taken into account in intestinal disorders. In our study, an investigation was conducted to determine the prevalence of intestinal micro-pathogens, such as Cryptosporidium, Giardia, Blastocystis, and microsporidia, in hospitalized patients with different immunological statuses. The study at hand indicates that protozoan parasitic infections are rare among immunodeficient patients in Poland. The overall prevalence of micro-pathogens among participants was 4.6%; it was three times higher in adults (12.5%) than in children (2.3%). Cryptosporidium and Cyclospora species (Apicomplexa) were diagnosed as the main cause of heavy diarrhea. Accordingly, adult patients were positive mainly for Blastocystis and microsporidia, while children were more often infected with the Cryptosporidium species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal opportunistic infections are caused by viruses, bacteria, or unicellular parasites. Patients with impaired immunity are particularly susceptible to infections which may develop into severe illness. The first symptoms of intestinal parasitic infections are diarrhea and other intestinal disorders, such as cramping abdominal pains, nausea, vomiting, or low-grade fever. Diarrhea is ordinarily chronic and prolonged in the course of opportunistic diseases (Pierce and Kirkpatrick 2009; Nimri and Meqdam 2004). It can lead to dehydration (Bednarska et al. 2015), weight loss (Kucik et al. 2004), or even death (Cheng et al. 2005). Cryptosporidium parvum and Cryptosporidium hominis are prevailing microparasites in patients with immunodeficiency (Khan et al. 2017; Fayer 2010; Bajer et al. 2008). As of today, approximately 30 species and genotypic variants of Cryptosporidium have been described in mammals (Siński et al. 1998; Bajer et al. 2011), birds (Helmy et al. 2017), reptiles (Paiva et al. 2013), amphibian (Jirků et al. 2008), and fish (Ryan 2010). Most human diseases are provoked by C. hominis and C. parvum species which can infect more than 100 species of mammals (Caccio et al. 2005). Some less common species typical for animals, such as Cryptosporidium meleagridis, Cryptosporidium felis, Cryptosporidium canis, Cryptosporidium muris, and Cryptosporidium suis, have been reported mainly in humans with immunodeficiency (Cacciò et al. 2002; Wolska-Kusnierz et al. 2007; Bajer et al. 2008; Xiao 2010). It should be highlighted that C. meleagridis, previously described only in Turkey, has been noted in 1% of cryptosporidiosis in the UK (Mosier and Oberst 2000) and 10–20% in Peru (Cama et al. 2008).
Other opportunistic parasite species, such as microsporidia, Cyclospora, Cystoisospora, and Blastocystis, may also be associated with gastrointestinal diseases. It is currently unclear whether Blastocystis is a pathogen, a commensal, or an opportunistic organism. In favorable conditions, it causes intestinal disorders, but the infection may be either self-limiting or asymptomatic (Tan 2004; Scanlan and Stensvold 2013).
Cyclospora and Cystoisospora are most commonly associated with diarrhea in travelers, especially those visiting endemic areas (Legua and Seas 2013). Parasitic infections may cause a significant problem in immunocompromised persons (very young, elderly, after transplantation, and with AIDS) (Forrest 2004; Lewthwaite et al. 2005; Barsoum 2004). Transplant recipients are more likely to suffer from parasitic invasions as a consequence of immunosuppressive therapy. In general, gastrointestinal infections have been increasingly reported in this risk group. There are a few epidemiological studies carried out worldwide to examine the intestinal parasitic infections in liver or renal transplant recipients (Azami et al. 2010; Batista et al. 2011; Bednarska et al. 2013, 2014; Krause et al. 2012).
Microsporidia are a group of pathogens still poorly recognized and diagnosed in a human population. Of the 15 microsporidia species identified as human pathogens, two species cause gastrointestinal disease: Enterocytozoon bieneusi and Encephalitozoon intestinalis—the former being more commonly identified in solid-organ transplant recipients (Anane and Attouchi 2010).
In our study, the prevalence of intestinal micro-pathogens in hospitalized patients with different immunological statuses is defined. Furthermore, the pathogenicity detected in the patients with Cryptosporidium spp. and Blastocystis hominis is discussed.
Materials and methods
Stool samples
The study was carried out in three specialized hospitals in Warsaw along with its surrounding area during 2007–2015. Fecal samples were collected in hospital wards by medical practitioners for the purposes of routine bacteriological examinations and, subsequently, subjected to further retrospective examinations. Written informed consent was obtained from all patients, and the study protocol followed ethical guidelines of the 2013 Declaration of Helsinki. All ethical approvals for the study have been obtained according to Polish regulations. Fresh stool samples were obtained on two, three, or more occasions from patients and stored at + 4 °C. Samples were obtained from 285 patients (121 male and 164 female) with different immune statuses in the following departments: Children’s Memorial Health Institute in Warsaw (CMHI): (1) Gastroenterology, Hepatology, Nutritional Disorders and Pediatrics Clinic (CZW) (n = 147; 58M/89F); (2) the Immunology Clinic (CZD) (n = 34; 20M/14F); (3) Pediatric Department in General Hospital in Otwock (OT) (n = 40; 13M/27F); (4) Department of Gastroenterology, Hepatology and Clinical Oncology, Medical Center for Postgraduate Education (ON) (n = 64; 30M/34F). The patients were subdivided into two groups according to their age (221 under and 64 above 18 years old) and three groups based on their immunological status (Table 1).
The first group involved 147 patients after liver transplantation under pharmacological immunosuppression [tacrolimus (TAC), sirolimus (SIR), cyclosporine (CSR) alone or collectively with steroids (ST), mycophenolate mofetil (MMF), or azathioprinum (AZP)]. The patients rarely manifested diarrhea or other intestinal disorders (6/147). The second group of children consisted of 34 patients, often with diarrhea (22/34), who presented impaired immunity due to confirmed (n = 32) or suspected (n = 2) primary immunodeficiencies (PID). The third group comprised 40 immunocompetent children with prolonged intestinal disorder (19/40) of unknown etiology. The fourth group consisted of 64 adult patients who presented acquired immunity disorders resulting from various acute diseases [Crohn’s disease (CD), colitis ulcerosa (UC), Clostridium difficile infection (CDI), Cytomegalovirus infections (CMV), rheumatoid arthritis (RAS), autoimmune enteropathy (AIE), hypereosinophilic syndrome (HES), common variable immunodeficiency (CVID), cholangiocarcinoma (CCC), unspecified immune resistance (UIR), radiotherapy (RTx)] and/or used drugs [glucocorticoids (GKS), AZP, 6-mercaptopurine (6-MP), MMF, Infliximabum (IFX)]. Most patients from this group presented prolonged diarrhea and/or other intestinal symptoms (54/64) often up to several months.
Staining of fecal smears
Fecal smears were made from fresh stool specimens, which were air-dried, fixed in methanol, and stained with Ziehl-Neelsen (AquaMed, Poland) for Cryptosporidium spp. This method is highly effective in Cyclospora cayetanensis detection. The modified Weber’s chromotrope-based staining, i.e. trichrome staining (Chromotrope 2R Para-Pak Trichrome Stain, Meridian Diagnostics, Cincinnati, OH, USA) (Weber et al. 1992), was used for the E. bieneusi and Encephalitozoon spp. diagnoses. Smears were examined under oil immersion (× 1000 magnification). Indirect immunofluorescence assay (IFA) was performed for the verification or detection of Cryptosporidium and/or Giardia infections (Merifluor Cryptosporidium/Giardia kit, Meridian Diagnostics, USA) and diagnosed by direct immunofluorescence microscopy (× 400 magnification).
PCR analysis
For DNA extraction, stool specimens were first concentrated by sedimentation (Bednarska et al. 2007). DNA extraction and purification were carried out using QIAamp DNA Stool Mini Kit (Qiagen), following the manufacturer’s protocol. Different sets of primers were used for PCR amplification with respect to the parasite species. A nested-PCR protocol was used to amplify the 18S rRNA gene fragments of Cryptosporidium spp. using primers previously described by Xiao et al. (1999). Additionally, a set of primers for Apicomplexa was used to confirm infection with C. felis (Herwaldt et al. 2003).
The next, “general” primers described by Raynaud et al. (1998) were used to amplify a 1200 bp conserved region of small-subunit ribosomal RNA genes (SSU-rDNA) with the aim of searching the range of human infecting microsporidial species, including Encephalitozoon cuniculi, Encephalitozoon hellem, E. intestinalis, and E. bieneusi. Species-specific primers were used to amplify a region of 545 bp from the SSU-rDNA of E. intestinalis (Valencáková et al. 2005), and species-specific primers were used to amplify a 607 bp fragment of the SSU-rDNA of E. bieneusi (da Silva et al. 1996).
Blastocystis hominis DNA was detected by PCR, previously described by Alfellani et al. (2013), to amplify the region of 600 bp from the SSU-rDNA.
Infection with C. cayetanensis was detected by microscopic methods and confirmed through nested PCR protocols used to amplify the 18S rRNA gene fragments using the published primer sets and thermal profiles. The nested PCR was performed to amplify a 500-bp fragment of C. cayetanensis 18S rDNA (Sulaiman et al. 2014).
All PCR products were subjected to electrophoresis in a 1.5% agarose gel stained with Midori Green stain (Nippon Genetics GmbH) and sequenced by a private company (Genomed S.A., Poland).
Statistical analysis
SPSS 21 software was used for analysis. Patients presenting with diarrhea were compared with those without such symptoms. By the same token, adults and minors were compared.
Both the correlation between the degree of suppression and the occurrence of invasion, as well as the occurrence of diarrhea and the number of parasitic infections, were analyzed.
Results
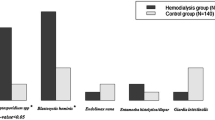
Out of the 283 patients (46 immunocompetent and 237 immunocompromised), a total of 5% (n = 14) were infected with intestinal parasites detected by microscopic, immunofluorescent, and/or PCR techniques. Additionally, three transplant recipients who were minors tested positive for E. coli bacteria strains which were closely related to enteroinvasive strains (99% homology) (Table 2).
The patients were infected with different Cryptosporidium species (1.4%, n = 4), Giardia intestinalis (0.7%, n = 2), C. cayetanensis (0.7%, n = 2), B. hominis (1%, n = 3), and presented with microsporidian invasion (n = 4). In one case, coinfection with Cyclospora and Blastocystis was detected (Table 2). The prevalence of pathogens was found in both immunocompetent (6.5%) and immunocompromised patients (4.6%). Micropathogen infections in children (< 18 years old, n = 221) and adults (> 18 years old, n = 62) were 3.2 and 12.9%, respectively (p = 0.226) (Table 3). There were significant differences in the prevalence of parasitic Protista (Cryptosporidium, Giardia, Cyclospora) between the male (5%) and female (0.6%) groups (p = 0.015, df = 1, χ2 = 5.885). The prevalence of Apicompexa infection with Cryptosporidium or Cyclospora species was significantly associated with diarrhea and heavy immunodeficient patients (p = 0.002, df = 2, χ2 = 12.88). There was an interesting link between micropathogen infections and immunosuppressed rates (p = 0.044, df = 2, χ2 = 6.242). Most parasitic infections were reported in patients with severe, second-stage immunodeficiency (6.1%), while in patients with mild or no immunosuppression, it was 0.6 and 2.2%, respectively.
Cryptosporidium infections
In our study, infections with Cryptosporidium occurred in four patients with diarrhea and heavy immunodeficiency. Among the four detected cases, three different species of Cryptosporidium were identified by PCR assay. Only one HIV+ adult patient (756/ON) was infected with C. parvum. Two prolonged infections in patients with PID (9/CZW, 17/CZW) were caused by C. meleagridis and Cryptosporidium spp., respectively (partially described by Wolska-Kusnierz et al. 2007). The infection caused by C. felis was detected in the liver transplant girl.
Our long-term study on two patients with prolonged cryptosporidiosis and heavy disorders was partially described by Wolska-Kusnierz et al. (2007). We reported the results of parasitological study, which was in progress for 7 years from 2007 to 2011–2014. Patient no. 9/04 infected with C. meleagridis underwent a four-time transplant in 2006 and, 6 years after transplantation with full immune reconstitution and no parasite infection, is alive and well. All results were obtained by three methods: ZN, IFA, and PCR—which at this time tested negative for Cryptosporidium.
Patient no. 17/05 with CD40 ligand deficiency complicated by cholangitis scleroticans and Cryptosporidium infection revealed Cryptosporidium infection at the age of 5, but long-term azithromycin treatment did not clear up or treat the infection. At age 7, he received matched unrelated stem cell transplantation. Liver failure with vanishing bile duct syndrome in the course of severe graft versus host disease (GVHD) occurred after transplantation. In March 2008, liver transplantation from an unrelated donor was successfully performed. In a follow-up study, we observed the clearance of Cryptosporidium infection together with full immune reconstitution. No recurrence of parasite infection was detected during the following 7 years of observation.
Blastocystis hominis infection
A partially retrospective study regarding B. hominis was carried out on 249 patients (187 minors). Positive PCR results were obtained only from adult patients (4.7%, 3/64), two of which were hospitalized. The 23-year-old male suffered from colitis ulcerosa, infections with Cytomegalovirus (CMV), and Clostridium difficile. The 41-year-old female complained of intestinal disorders, such as abdominal pain, weight loss, and alternating rhythm of bowel movements. The third patient (PC2), infected with Blastocystis, was first diagnosed with C. cayetanensis. Coinfection with Blastocystis was detected based on the molecular study. Three defecations per day were reported as a physiological norm by this immunocompetent male (Bednarska et al. 2015).
DNA sequence alignments and phylogenetic analysis were conducted using MEGA version 6.0. Two isolates from 758/ON and PC2/ON patients were closely related (identical), and both were grouped in the region (III) closely related to human isolates. The nucleotide sequences of the ITS fragment of DNA isolated from patient no. 764/ON were also related to genotypes rarely isolated from human specimens (region II) (Abe 2004). The relatedness of isolates, grouped by its sequence identity, is showed in the phylogenetic tree (Fig. 1). The GenBank accession numbers assigned to the sequences determined in this study are as follows: genotype 758/ON, MG905018, genotype 764/ON, MG905016, and genotype PC2/ON, MG905017.
Evolutionary relationships of taxa. The evolutionary history was inferred using the Neighbor-Joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura2 parameter method and are in the units of the number of base substitutions per site. The analysis involved 51 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 398 positions in the final data set. Evolutionary analyses were conducted in MEGA6
Discussion
Prevalence of micro-parasitic infection
Microparasites, such as viruses and bacteria, may cause infective diarrhea in immunodeficient and post-transplant patients in endemic areas, yet the data regarding such infections in Poland are scarce (Table 4). The research presented here represents one of few studies of the incidence, disease manifestation, management, and outcome of microparasitic infection in transplant recipients and immunocompromised individuals. This study indicates that protozoan parasitic infections are rare among immunodeficient patients in Poland. The overall prevalence of micro-pathogens in the study participants was 4.6%, and it was three times higher in adults (12.5%) than in children (2.3%), which attests to the fact that microparasitic infections are the most frequent in patients who are not minors. Accordingly, adult patients tested positive mainly for Blastocystis and microsporidia, while children were more often infected with the Cryptosporidium species. It is worthy of attention that Cryptosporidium parasites, together with G. intestinalis, were included in the WHO’s “Neglected disease initiative” in 2004 (Savioli et al. 2006) due to their significance in public health.
Detection and characterization of Cryptosporidium spp. and Blastocystis spp. isolates
It is known from earlier studies that cryptosporidiosis prevalence varies from 1 to 5% in children from developed countries and 50% in children from developing countries. Cryptosporidiosis can be found more often in young children and immunocompromised patients (especially those with HIV-associated immunosuppression) than in a healthy adult population (Cheng et al. 2005).
In our study, the Cryptosporidium species was detected in 3.6% of children, which is similar to the 1.2% in the Teheran study (Tahvildar-Biderouni and Salehi 2014), 2% in Poland (Solarczyk et al. 2010) 3.9% of the Cryptosporidium-infected children in the study of Tanzania (Tellevik et al. 2015), 4.6% in Ethiopia (de Lucio et al. 2016), and 2.4% Iranian children (Taghipour et al. 2011). Cryptosporidium prevalence found in this study is lower than that reported for children with diarrhea in Canada which was at 15.7% (Iqbal et al. 2015), 10.4% in Tanzania, and 15.1% in Qatar (Boughattas et al. 2017). These variations could be explained by the differences in the region of study, the hygiene practices, as well as the socio-economic status of participants involved in the studies.
By using molecular methods in this study, C. parvum, C. meleagridis, and C. felis were identified in diarrheic patients. All identified Cryptosporidium isolates are considered as zoonotic species which are commonly reported in humans and wildlife worldwide (Xiao 2010).
The distribution of Cryptosporidium species in humans varies across geographic areas and socioeconomic conditions (Chalmers and Katzer 2013). In European countries, the detection of infection with C. parvum, C. hominis, and C. meleagridis in humans increased. Infections with C. canis and C. felis are reported in studies conducted in developing countries (Xiao 2010).
In our study, two cases of chronic cryptosporidiosis (C. meleagridis and Cryptosporidium sp.) were reported among PID children. Hyper-IgM patients with C. meleagridis infection (partially reported by Wolska-Kusnierz et al. 2007) were monitored after four bone marrow transplantations (the last one performed in 2006), and the problem with cryptosporidiosis was resolved. Within a few years, no relapse to Cryptosporidium infection was observed. The resolution of opportunistic infections in immune-suppressed patients requires the restoration of mucosal immunity, usually achieved following the discontinuation of immunosuppressive drugs (Nachbaur et al. 1997). The patient diagnosed with CD40 ligand deficiency, sclerosis cholangitis, and prolonged cryptosporidiosis required bone marrow transplant as well as liver transplant after GVHD intended for the full immune reconstruction. Chronic cryptosporidiosis can cause GVHD after stem cell transplantation, thus requiring a reduction of immunosuppressive drugs and a specific therapy, whereas GVHD requires intensification of immunosuppression (Legrand et al. 2011; Washington and Jagasia 2009). Presumably, in the case in question, the heavy immunosuppression led to re-development of cryptosporidiosis. Relapse to cryptosporidiosis at this stage of treatment can be dangerous for the health and life of a patient. Therefore, control and prompt diagnosis of intestinal cryptosporidiosis are recommended.
Cryptosporidium felis has a visibly more restricted host range than C. parvum and, using molecular techniques, it has been confirmed that it may infect cats (Lucio-Forster et al. 2010), immunocompetent and immunocompromised humans (Cacciò et al. 2002), as well as cattle (Bornay-Llinares et al. 1999). Infection with C. felis was detected in a young female who had undergone a liver transplant. Importantly, this patient resided in a rural environment with direct access to dogs, cats, and other farm animals. The most probable source of infection comprised cats from the close surroundings. In children from developing countries, C. felis is responsible for as much as 3.3% of all cryptosporidiosis cases (Lucio-Forster et al. 2010).
Cryptosporidium infections were detected aided by microscopic studies, using Ziehl-Neelsen staining and the IFA method, while the species identification was confirmed using PCR. Interestingly, in this case, C. felis infection was not detected in immunofluorescent testing dedicated to detection of a wide range of Cryptosporidium species in stool specimens. These results suggest that studies on the transmission of zoonotic species are difficult due to the lack of suitable subtyping tools for the distinction of Cryptosporidium spp. (Ryan et al. 2014). Given the above, it is necessary that two different methods for the detection of Cryptosporidium and other parasitic infections in humans are employed. A diagnosis based only on a microscopic or immunofluorescent test or molecular methods only may lead to false-negative results. A molecular study is necessary to recognize the genotype and subtype of Cryptosporidium and to identify the organism responsible for infection along with the source and routes of transmission.
Infection with Blastocystis has been reported as asymptomatic, acute, or chronic symptomatic (Windsor et al. 2002; Tan 2004). This wide range of responses to infection could be related to genetic diversity. Blastocystis hominis was detected only in adult patients (4.7%), and in one male patient, co-infection with C. cayetanensis was found. In this study, the pathogen was detected only by molecular methods. An earlier study of patients with hematopoietic and lymphoid hyperplastic diseases (not published) compared light microscopy, immunofluorescence, ELISA, cultivation using Joni’s medium, and PCR as methods used for detection of Blastocystis sp. The most useful diagnostic methods seem to be cultivation (15%, 6/40) and PCR (17.5%, 7/40). Parasites were detected in 30% of patients using both above-mentioned methods. These results confirm that two diagnostic methods should be used in parasitological diagnostic.
The sources of infection with Blastocystis can be water or zoonotic transmission (Abe 2004), while the risk factors include immunocompromised health (Rao et al. 2003) or poor hygiene practices (Nimri and Meqdam 2004; Tan 2004). Pathogenicity of Blastocystis is controversial and still undefined. Thus, further research should be carried out to determine the potential risk associated with the invasiveness of their subtypes. The most dominant subtypes in humans are subtype 3 (41.7 to 92.3%) and subtype 1 (7.7 to 25%), followed by either subtype 6 (10 to 22.9%), subtype 2 (1.3 to 32.1%), or subtype 4 (1.3 to 37.5%)—the occurrence of which is associated with geographical distributions. In most studies, other genotypes (ST5, ST7–9) were identified at lower frequencies globally (Tan 2004; Alfellani et al. 2013). In our study, subtype 2 was detected in immunodeficient patients with colitis ulcerosa and co-infections with CMV and C. difficile. Subtype ST-3 was diagnosed in two immunocompetent patients, one of which was hospitalized due to weight loss and alternating rhythm of bowel movements. The other one was diagnosed with co-invasion during the prolonged, asymptomatic infection with C. cayetanensis (Bednarska et al. 2015). It is difficult to assess the impact of B. hominis infection on the chronic C. cayetanensis infection. The higher frequency of defecations in this patient could have been a symptom of irritable bowel caused by Blastocystis. Further research must be conducted to clarify the effects of B. hominis on intestinal peristalsis, asymptomatic C. cayetanensis infection, or any different microparasitic infection which may result in prolonged contamination of the environment by asymptomatic, but chronically infected patients.
Diarrhea in immunodeficiency
A majority of diarrhea cases were due to non-parasitic infections. In total, 35% patients had symptoms at the time of the survey. Gastrointestinal symptoms were more often reported in children with PID (65%) and adult patients (84%). Post-transplant diarrhea is a common and distressing occurrence in patients, which can have significant deleterious effects on the clinical course and well-being of organ recipients. The true incidence of diarrhea in liver transplant recipients is unknown but possibly ranges from 10 to 43%—according to published studies in other solid organ and bone marrow transplantation (Azami et al. 2010; Galván et al. 2011; Agholi et al. 2013). Our observations did not agree with these data. In our study on liver transplant recipients, only 2% were infected with parasites, but diarrhea was occasionally presented (4%). Diarrhea could be a frequent side effect of immunosuppressive medications (mycophenolate mofetil (MMF), cyclosporine A (CSA), tacrolimus, and sirolimus) or an additional infectious agent, including viruses (e.g. Cytomegalovirus), bacteria, or fungi (e.g. C. difficile) (Bonatti et al. 2012; Song et al. 2006; Dave et al. 2014). More than half of the patients tested in this study had heavy immunodeficiency due to medications or diseases such as inflammatory bowel disease (e.g., colitis ulcerosa) or celiac disease, and probably these factors were the main reasons for intestinal disorders.
In conclusion, the current study illustrates the need to maintain a high index of suspicion for microparasites, especially Cryptosporidium, microsporidia, and Blastocystis in immunodeficient or transplant patients who present prolonged diarrhea. We diagnosed Cryptosporidium and Cyclospora species (both Apicomplexa) as the main cause of heavy parasitic diarrhea. In our opinion, parasitic infections should be diagnosed with two different methods for an accurate diagnosis. Probably, the routine stool evaluations for parasites may not identify rare zoonotic species or low intensity of parasites. Furthermore, our results imply that a molecular analysis used to identify the parasite species should be performed as soon as the zoonotic Cryptosporidium infection is suspected. Various other etiologies, including inflammatory bowel disease, must be considered in the differential diagnosis. This will allow choosing the proper treatment for specific parasite infections.
References
Abe N (2004) Molecular and phylogenetic analysis of Blastocystis isolates from various hosts. Vet Parasitol 120:235–242
Agholi M, Hatam GR, Motazedian MH (2013) Microsporidia and coccidia as causes of persistence diarrhea among liver transplant children: incidence rate and species/genotypes. Pediatr Infect Dis J 32:185–187
Alfellani MA, Jacob AS, Perea NO, Krecek RC, Taner-Mulla D, Verweij JJ, Levecke B, Tannich E, Clark CG, Stensvold CR (2013) Diversity and distribution of Blastocystis sp. subtypes in non-human primates. Parasitology 140:966–971
Anane S, Attouchi H (2010) Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterol Clin Biol 34:450–464
Azami M, Sharifi M, Hejazi SH, Tazhibi M (2010) Intestinal parasitic infections in renal transplant recipients. Braz J Infect Dis 14:15–18
Bajer A, Bednarska M, Cacciò SM, Wolska-Kuśnierz B, Heropolitanska-Pliszka E, Bernatowska E, Wielopolska M, Paziewska A, Welc-faleciak R, Siński E (2008) Genotyping of Cryptosporidium isolates from human clinical cases in Poland. Parasitol Res 103:37–42
Bajer A, Bednarska M, Rodo A (2011) Risk factors and control of intestinal parasite infections in sled dogs in Poland. Vet Parasitol 175:343–350
Barsoum RS (2004) Parasitic infections in organ transplantation. Exp Clin Transplant 2:258–267
Batista MV, Pierrotti LC, Abdala E, Clemente WT, ES G˜o, Rosa DR, Ianhez LE, Bonazzi PR, Lima AS, Fernandes PF, Padua-Neto MV, Bacchella T, Oliveira AP, Viana CF, Ferreira MS, Shikanai-Yasuda MA (2011) Endemic and opportunistic infections in Brazilian solid organ transplant recipients. Tropical Med Int Health 16:1134–1142
Bednarska M, Bajer A, Sinski E, Girouard AS, Tamang L, Graczyk TK (2007) Fluorescent in situ hybridization as a tool to retrospectively identify Cryptosporidium parvum and Giardia lamblia in samples from terrestrial mammalian wildlife. Parasitol Res 100:455–460
Bednarska M, Bajer A, Welc-Faleciak R, Czubkowski P, Teisseyre M, Graczyk TM, Jankowska I (2013) First case of Enterocytozoon bieneusi infection in Poland. Ann Agric Environ Med 20:287–288
Bednarska M, Bajer A, Siński E, Wolska-Kuśnierz B, Samoliński B, Graczyk TK (2014) The occurrence of intestinal microsporidia in immunodeficient patients in Poland. Ann Agric Environ Med 21:244–248
Bednarska M, Bajer A, Welc-Falęciak R, Pawełas A (2015) Cyclospora cayetanensis infection in transplant traveller: a case report of outbreak. Parasit Vectors 8:411
Bonatti H, Barroso LF 2nd, Sawyer RG, Kotton CN, Sifri CD (2012) Cryptosporidium enteritis in solid organ transplant recipients: multicenter retrospective evaluation of 10 cases reveals an association with elevated tacrolimus concentrations. Transpl Infect Dis 14:635–648
Bornay-Llinares FJ, da Silva AJ, Moura IN, Myiak P, Pietkiewicz H, Kruminis-Lozowka W, Graczyk TK, Pieniazek NJ (1999) Identification of Cryptosporidium felis in a cow by morphologic and molecular methods. Appl Environ Microbiol 65:1455–1458
Boughattas S, Behnke JM, Al-Ansari K, Sharma A, Abu-Alainin W, Al-Thani A, Abu-Madi MA (2017) Molecular analysis of the enteric protozoa associated with acute diarrhea in hospitalized children. Front Cell Infect Microbiol 7:343
Cacciò S, Pinter E, Fantini R, Mezzaroma I, Pozio E (2002) Human infection with Cryptosporidium felis: case report and literature review. Emerg Infect Dis 8:85–86
Caccio SM, Thompson RC, Mclauchlin J, Smith HV (2005) Unraveling Cryptosporidium and Giardia epidemiology. Trends Parasitol 21:430–437
Cama VA, Bern C, Roberts J, Cabrera L, Sterling CR, Ortega Y, Gilman RH, Xiao L (2008) Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg Infect Dis 14:1567–1574
Chalmers RM, Katzer F (2013) Looking for Cryptosporidium: the application of advances in detection and diagnosis. Trends Parasitol 29:237–251
Cheng AC, McDonald JRMD, Nathan M (2005) Infectious diarrhoea in developed and developing countries. J Clin Gastroenterol 39:757–773
da Silva AJ, Schwartz DA, Visvesvara GS, de Moura H, Slemenda SB, Pieniazek NJ (1996) Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol 34:986–987
Dave M, Purohit T, Razonable R, Loftus EV Jr (2014) Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis 20:196–212
de Lucio A, Amor-Aramendía A, Bailo B, Saugar JM, Anegagrie M, Arroyo A, López-Quintana B, Zewdie D, Ayehubizu Z, Yizengaw E, Abera B, Yimer M, Mulu W, Hailu T, Herrador Z, Fuentes I, Carmena D (2016) Prevalence and genetic diversity of Giardia duodenalis and Cryptosporidium spp. among school children in a rural area of the Amhara region, north-west Ethiopia. PLoS One 11:e0159992
Duda A, Kosik-Bogacka D, Lanocha-Arendarczyk N, Kołodziejczyk L, Lanocha A (2015) The prevalence of Blastocystis hominis and other protozoan parasites in soldiers returning from peacekeeping missions. Am J Trop Med Hyg 92:805–806
Fayer R (2010) Taxonomy and species delimitation in Cryptosporidium. Exp Parasitol 124(1):90–97
Forrest G (2004) Gastrointestinal infections in immunocompromised hosts. Curr Opin Gastroenterol 20:16–21
Galván AL, Sánchez AM, Valentín MA, Henriques-Gil N, Izquierdo F, Fenoy S, del Aguila C (2011) First cases of microsporidiosis in transplant recipients in Spain and review of the literature. J Clin Microbiol 49:1301–1306
Helmy YA, Krücken J, Abdelwhab EM, von Samson-Himmelstjerna G, Hafez HM (2017) Molecular diagnosis and characterization of Cryptosporidium spp. in turkeys and chickens in Germany reveals evidence for previously undetected parasite species. PLoS One 12:e0177150. https://doi.org/10.1371/journal.pone.0177150
Herwaldt BL, Cacciò S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga P, Martinelli G, Edelhofer R, Hollenstein U, Poletti G, Pampiglione S, Löschenberger K, Tura S, Pieniazek NJ (2003) Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis 9:942–948
Iqbal A, Goldfarb DM, Slinger R, Dixon BR (2015) Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in diarrhoeic patients in the Qikiqtani Region, Nunavut, Canada. Int J Circumpolar Health 74:27713
Jirků M, Valigurová A, Koudela B, Krízek J, Modrý D, Slapeta J (2008) New species of Cryptosporidium Tyzzer, 1907 (Apicomplexa) from amphibian host: morphology, biology and phylogeny. Folia Parasitol (Praha) 55:81–94
Khan A, Shaik JS, Grigg ME (2017) Genomics and molecular epidemiology of Cryptosporidium species. Acta Trop 184:1–14. https://doi.org/10.1016/j.actatropica.2017.10.023
Kicia M, Wesolowska M, Jakuszko K, Kopacz Z, Sak B, Květonová D, Krajewska M, Kváč M (2014) Concurrent infection of the urinary tract with Encephalitozoon cuniculi and Enterocytozoon bieneusi in a renal transplant recipient. J Clin Microbiol 52:1780–1782. https://doi.org/10.1128/JCM.03328-13
Kłudkowska M, Pielok Ł, Frąckowiak K, Paul M (2017) Intestinal coccidian parasites as an underestimated cause of travellers' diarrhoea in Polish immunocompetent patients. Acta Parasitol 62:630–638
Krause I, Amir J, Cleper R, Dagan A, Behor J, Samra Z, Davidovits M (2012) Cryptosporidiosis in children following solid organ transplantation. Pediatr Infect Dis J 31:1135–1138
Kucik CJ, Martin GL, Sortor BV (2004) Common intestinal parasites. Am Fam Physician 69:1161–1168
Legrand F, Grenouillet F, Larosa F, Dalle F, Saas P, Millon L, Deconinck E, Rohrlich PS (2011) Diagnosis and treatment of digestive cryptosporidiosis in allogeneic haematopoietic stem cell transplant recipients: a prospective single centre study. Bone Marrow Transplant 46:858–862
Legua P, Seas C (2013) Cystoisospora and Cyclospora. Curr Opin Infect Dis 26:479–483
Lewthwaite P, Gill GV, Hart CA, Beeching NJ (2005) Gastrointestinal parasites in the immunocompromised. Curr Opin Infect Dis 18:427–435
Lucio-Forster A, Griffiths JK, Cama VA, Xiao L, Bowma DD (2010) Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol 26:174–179
Mosier DA, Oberst RD (2000) Cryptosporidiosis. A global challenge. Annals New York Academy Sci 916:102–111
Nachbaur D, Kropshofer G, Feichtinger H, Allerberger F, Niederwieser D (1997) Cryptosporidiosis after CD34 selected autologous peripheral blood stem cell transplantation (PBSCT) treatment with paromomycin, azithromycin and recombinant human interleukin-2. Bone Marrow Transplant 19:1261–1263
Nimri LF, Meqdam M (2004) Enteropathogens associated with cases of gastroenteritis in a rural population in Jordan. Clin Microbiol Infect 10:634–639
Paiva PR, Grego KF, Lima VM, Nakamura AA, da Silva DC, Meireles MV (2013) Clinical, serological, and parasitological analysis of snakes naturally infected with Cryptosporidium serpentis. Vet Parasitol 198(1–2):54–61. https://doi.org/10.1016/j.vetpar.2013.08.016
Pierce KK, Kirkpatrick BD (2009) Update on human infections caused by intestinal protozoa. Curr Opin Gastroenterol 25:12–17
Rao K, Sekar U, Iraivan KT, Abraham G, Soundararajan P (2003) Blastocystis hominis—an emerging cause of diarrhoea in renal transplant recipients. J Assoc Physicians India 51:719–721
Raynaud L, Delbac F, Broussolle V, Rabodonirina M, Girault V, Wallon M, Cozon G, Vivares CP, Peyron F (1998) Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J Clin Microbiol 36:37–40
Rożej W, Gołab E, Waloch M, Wąsik M, Sadkowska-Todys M, Czerwiński M, Dzbenski TH (2010) The occurrence of Cryptosporidium in a group of children and adults with diarrhoea of undetermined earlier aetiology. Przegl Epidemiol 64:35–39 Polish
Ryan U (2010) Cryptosporidium in birds, fish and amphibians. Exp Parasitol 124:113–120. https://doi.org/10.1016/j.exppara.2009.02.002
Ryan U, Fayer R, Xiao L (2014) Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology 141:1667–1685
Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 22:203–208
Scanlan PD, Stensvold CR (2013) Blastocystis: getting to grips with our guileful guest. Trends Parasitol 29:523–529
Siński E, Bednarska M, Bajer A (1998) The role of wild rodents in ecology of cryptosporidiosis in Poland. Folia Parasitol (Praha) 45:173–174
Solarczyk P, Werner A, Majewska AC (2010) Genotype analysis of Giardia duodenalis isolates obtained from humans in west-central Poland. Ann Parasitol 56:171–177 Polish
Song ATWS, Abdala E, Bonazzi PR, Bacchella T, Machado MC (2006) Does mycophenolate mofetil increase the risk of cytomegalovirus infection in solid organ transplant recipients?: a mini review. Braz J Infect Dis 10:132–138
Sulaiman IM, Ortega Y, Simpson S, Kerdahi K (2014) Genetic characterization of human-pathogenic C. cayetanensis parasites from three endemic regions at the 18Sribosomal RNA locus. Infect Genet Evol 22:229–234
Taghipour N, Nazemalhosseini-Mojarad E, Haghighi A, Rostami-Nejad M, Romani S, Keshavarz A, Alebouyeh M, Zali M (2011) Molecular epidemiology of cryptosporidiosis in Iranian children, Tehran, Iran. Iranian J Parasitol 6:41–45
Tahvildar-Biderouni F, Salehi N (2014) Detection of Cryptosporidium infection by modified Zeihl-Neelsen and PCR methods in children with diarrheal samples in pediatric hospitals in Tehran. Gastroenterol Hepatol Bed Bench 7:125–130
Tan K (2004) Review Blastocystis in humans and animals: new insights using modern methodologies. Vet Parasitol 126:121–144
Tellevik MG, Moyo SJ, Blomberg B, Hjøllo T, Maselle SY, Langeland N, Hanevik K (2015) Prevalence of Cryptosporidium parvum/hominis, Entamoeba histolytica and Giardia lamblia among young children with and without diarrhea in Dar es Salaam, Tanzania. PLoS Negl Trop Dis 9(10):e0004125
Valencáková A, Bálent P, Novotný F, Cisláková L (2005) The diagnosis of Encephalitozoon spp. Ann Agric Environ Med 12:321–323
Washington K, Jagasia M (2009) Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol 40:909–917
Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS (1992) Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The enteric opportunistic infections working group. N Engl J Med 16:161–166
Wesołowska M, Szostakowska B, Kicia M, Sak B, Kvac M, Knysz B (2016) Cryptosporidium meleagridis infection: the first report in Poland of its occurrence in an HIV-positive woman. Ann Parasitol 62:239–241. https://doi.org/10.17420/ap6203.58
Windsor JJ, Macfarlane L, Hughes-Thapa G, Jones SA, Whiteside TM (2002) Incidence of Blastocystis hominis in faecal samples submitted for routine microbiological analysis. Br J Biomed Sci 59:154–157
Wolska-Kusnierz B, Bajer A, Caccio S, Heropolitanska-Pliszka E, Bernatowska E, van Dongen J, Bednarska M, Paziewska A, Siński E (2007) Cryptosporidium infection in patients with primary immunodeficiency syndromes. J Pediatr Gastroenterol Nutr 45:458–164
Xiao L (2010) Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol 124:80–89
Xiao L, Escalante L, Yang C, Sulaiman I, Escalante AA, Montali RJ, Fayer R, Lal AA (1999) Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNAgene locus. Appl Environ Microbiol 65:1578–1583
Acknowledgments
This study was partially supported by the Ministry of Science and Higher Education in Warsaw, Poland (Grant No. N N404101036).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Written informed consent was obtained from all patients or guardians of minor patients, and the study protocol followed ethical guidelines of the 2013 Declaration of Helsinki. All approvals for the study have been obtained according to Polish regulations.
Competing interests
The authors declare that they have no competing interests.
Additional information
Section Editor: Kevin S.W. Tan
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bednarska, M., Jankowska, I., Pawelas, A. et al. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol Res 117, 2869–2879 (2018). https://doi.org/10.1007/s00436-018-5976-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5976-6