Abstract
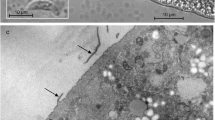
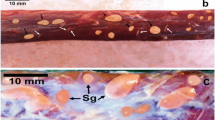
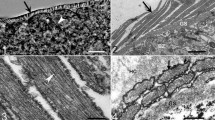
In the present study, we describe Sarcocystis entzerothi n. sp. from the European roe deer (Capreolus capreolus) based on the microscopical and DNA analysis. By light microscopy (LM), cysts of S. entzerothi were spindle-shaped with pointed tips, 950–1900 × 70–150 μm in size and had 5–6 μm long finger-like cyst wall protrusions. Cyst wall of S. entzerothi by transmission electron microscopy (TEM) was type 10a-like; villar protrusions were up to 1.2 μm wide, densely packed, lying about 0.1 μm between each other, had profuse microgranules and microfilaments, parasitophorous vacuolar membrane had many minute invaginations, and the ground substance layer measured up to 0.4 μm. This species is morphologically similar to Sarcocystis silva, previously found in the roe deer and the moose (Alces alces). By LM, cysts of S. silva were cigar-shaped with blunted tips, measured 1000–1500 × 130–184 μm, and had 7–8 μm long finger-like cyst wall protrusions. Under TEM, S. silva had no clear differences from S. entzerothi in their cyst wall ultrastructure. Having examined six roe deer hunted in Lithuania, cysts of S. entzerothi and S. silva were identified in four and two animals, respectively. These two Sarcocystis species could be morphologically differentiated according to the shape of the cysts and the length of protrusions. The species examined showed 95.6–96.1 % and 85.6–86.9 % sequence identity within 18S ribosomal DNA (rDNA) and cox1, respectively, and therefore they could be clearly distinguished by means of molecular methods. It should be noted that in the 18S rDNA phylogenetic tree, S. entzerothi from the roe deer was placed together with one sequence of Sarcocystis sp. from the Lithuanian red deer (Cervus elaphus) demonstrating the same species. Based on 18S rDNA and cox1 sequences, S. entzerothi was more closely related to Sarcocystis species transmitted via felids than canids.




Similar content being viewed by others
References
Atkinson CT, Wright SD, Telford SR, Mclaughlin GS, Forrester DJ, Roelke ME, Mccown JW (1993) Morphology, prevalence, and distribution of Sarcocystis spp. in white-tailed deer (Odocoileus virginianus) from Florida. J Wildl Dis 29:73–84. doi:10.7589/0090-3558-29.1.73
Bergmann V, Kinder E (1976) Elektronenmikroskopische Untersuchungen zur Wandstruktur von Sarkozysten in der Skelettmuskulatur von Wildschwein und Reh. Monatsh Vet Med 31:785–788
Blažek K, Schramlová J, Ippen R (1978) Dog as definitive host of sarcosporidia infecting roe deer. Folia Parasitol 25:95–96
Calero-Bernal R, Verma SK, Cerqueira-Cézar CK, Schafer LM, Van Wilpe E, Dubey JP (2015) Sarcocystis mehlhorni, n. sp. (Apicomplexa: Sarcocystidae) from the black-tailed deer (Odocoileus hemionus columbianus). Parasitol Res 114:4397–4403. doi:10.1007/s00436-015-4679-5
Colwell DD, Mahrt JL (1981) Ultrastructure of the cyst wall and merozoites of Sarcocystis from moose (Alces alces) in Alberta, Canada. Z Parasitenkd 65:317–329. doi:10.1007/bf00926727
Dahlgren SS, Gjerde B (2007) Genetic characterisation of six Sarcocystis species from reindeer (Rangifer tarandus tarandus) in Norway based on the small subunit rRNA gene. Vet Parasitol 146:204–213. doi:10.1016/j.vetpar.2007.02.023
Dahlgren SS, Gjerde B (2008) Sarcocystis in moose (Alces alces): molecular identification and phylogeny of six Sarcocystis species in moose, and a morphological description of three new species. Parasitol Res 103:93–110. doi:10.1007/s00436-008-0936-1
Dahlgren SS, Gjerde B (2009) Sarcocystis in Norwegian roe deer (Capreolus capreolus): molecular and morphological identification of Sarcocystis oviformis n. sp. and Sarcocystis gracilis and their phylogenetic relationship with other Sarcocystis species. Parasitol Res 104:993–1003. doi:10.1007/s00436-008-1281-0
Dahlgren SS, Gjerde B (2010) Molecular characterization of five Sarcocystis species in red deer (Cervus elaphus), including Sarcocystis hjorti n. sp., reveals that these species are not intermediate host specific. Parasitology 137:815–840. doi:10.1017/S0031182009991569
Dahlgren SS, Gouveia-Oliveira R, Gjerde B (2008) Phylogenetic relationships between Sarcocystis species from reindeer and other Sarcocystidae deduced from ssu rRNA gene sequences. Vet Parasitol 151:27–35. doi:10.1016/j.vetpar.2007.09.029
Dubey JP (1980) Sarcocystis species in moose (Alces alces), bison (Bison bison), and pronghorn (Antilocapra americana) in Montana. Am J Vet Res 41:2063–2065
Dubey JP, Lozier SC (1983) Sarcocystis infection in the white-tailed deer (Odocoileus virginianus) in Montana: intensity and description of Sarcocystis odoi n. sp. Am J Vet Res 44:1738–1743
Dubey JP, Speer CA (1985) Prevalence and ultrastructure of three types of Sarcocystis in mule deer, Odocoileus hemionus (Rafinesque), in Montana. J Wildl Dis 21:219–228. doi:10.7589/0090-3558-21.3.219
Dubey JP, Speer CA (1986) Sarcocystis infections in mule deer (Odocoileus hemionus) in Montana and the description of three new species. Am J Vet Res 47:1052–1055
Dubey JP, Jolley WR, Thorne ET (1983) Sarcocystis sybillensis sp. nov. from the North American elk (Cervus elaphus). Can J Zool 61:737–742. doi:10.1139/z83-098
Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R (2015) Sarcocystosis of animals and humans, 2nd edn. CRC Press, Boca Raton
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi:10.1093/nar/gkh340
Entzeroth R (1981) Untersuchungen an Sarkosporidien (Mieschersche Schläuche) des einheimischen Rehwildes (Capreolus capreolus L). Z Jagdwiss 27:247–257. doi:10.1007/BF02243674
Entzeroth R (1982) A comparative light and electron microscope study of the cysts of Sarcocystis species of roe deer (Capreolus capreolus). Z Parasitenkd 66:281–292. doi:10.1007/bf00925345
Entzeroth R (1985) Light-, scanning-, and transmission electron microscope study of the cyst wall of Sarcocystis gracilis Rátz, 1909 (Sporozoa, Coccidia) from the roe deer (Capreolus capreolus L.). Arch Protistenkd 129:183–186. doi:10.1016/s0003-9365(85)80020-8
Entzeroth R, Scholtyseck E, Greuel E (1978) The roe deer intermediate host of different Coccidia. Naturwissenschaften 65:395. doi:10.1007/bf00439714
Entzeroth R, Nemeséri L, Scholtyseck E (1983) Prevalence and ultrastructure of Sarcocystis sp. from the red deer (Cervus elaphus L.) in Hungary. Parasitol Hung 16:47–52
Entzeroth R, Chobotar B, Scholtyseck E, Neméseri L (1985) Light and electron microscope study of Sarcocystis sp. from the fallow deer (Cervus dama). Z Parasitenkd 71:33–39. doi:10.1007/bf00932916
Erber M, Boch J, Barth D (1978) Drei Sarkosporidienarten des Rehwildes. Berl Münch Tierärztl Wsch 91:482–486
Gjerde B (1985) Ultrastructure of the cysts of Sarcocystis grueneri from cardiac muscle of reindeer (Rangifer tarandus tarandus). Z Parasitenkd 71:189–198. doi:10.1007/BF00926269
Gjerde B (1986) Scanning electron microscopy of the sarcocysts of six species of Sarcocystis from reindeer (Rangifer tarandus tarandus). Acta Pathol Microbiol Immunol Scand [B] 94:309–317. doi:10.1111/j.1699-0463.1986.tb03058.x
Gjerde B (2012) Morphological and molecular characterization and phylogenetic placement of Sarcocystis capreolicanis and Sarcocystis silva n. sp. from roe deer (Capreolus capreolus) in Norway. Parasitol Res 110:1225–1237. doi:10.1007/s00436-011-2619-6
Gjerde B (2013) Phylogenetic relationships among Sarcocystis species in cervids, cattle and sheep inferred from the mitochondrial cytochrome c oxidase subunit I gene. Int J Parasitol 43:579–591. doi:10.1016/j.ijpara.2013.02.004
Gjerde B (2014a) Morphological and molecular characteristics of four Sarcocystis spp. in Canadian moose (Alces alces), including Sarcocystis taeniata n. sp. Parasitol Res 113:1591–1604. doi:10.1007/s00436-014-3806-z
Gjerde B (2014b) Sarcocystis species in red deer revisited: with a redescription of two known species as Sarcocystis elongata n. sp. and Sarcocystis truncata n. sp. based on mitochondrial cox1 sequences. Parasitology 141:441–452. doi:10.1017/S0031182013001819
Gjerde B (2016a) Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol Res 115:1473–1492. doi:10.1007/s00436-015-4881-5
Gjerde B (2016b) The resurrection of a species: Sarcocystis bovifelis Heydorn et al., 1975 is distinct from the current Sarcocystis hirsuta in cattle and morphologically indistinguishable from Sarcocystis sinensis in water buffaloes. Parasitol Res 115:1–21. doi:10.1007/s00436-015-4785-4
Gjerde B, Hilali M (2016) Domestic cats (Felis catus) are definitive hosts for Sarcocystis sinensis from water buffaloes (Bubalus bubalis). J Vet Med Sci. doi:10.1292/jvms.16-0127
Hernández-Rodríguez S, Martínez-Gómez F, Navarrete I, Acosta-García I (1981) Estudio al microscopio optico y electronico del quiste de Sarcocystis cervicanis. Rev Ibérica Parasitol 41:351–361
Holmdahl OJ, Morrison DA, Ellis JT, Huong LT (1999) Evolution of ruminant Sarcocystis (Sporozoa) parasites based on small subunit rDNA sequences. Mol Phylogenet Evol 11:27–37. doi:10.1006/mpev.1998.0556
Jeffries AC, Schnitzler B, Heydorn AO, Johnson AM, Tenter AM (1997) Identification of synapomorphic characters in the genus Sarcocystis based on 18S rDNA sequence comparison. J Eukaryot Microbiol 44:388–392. doi:10.1111/j.1550-7408.1997.tb05713.x
Kolenda R, Ugorski M, Bednarski M (2014) Molecular characterization of Sarcocystis species from Polish roe deer based on ssu rRNA and cox1 sequence analysis. Parasitol Res 113:3029–3039. doi:10.1007/s00436-014-3966-x
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi:10.1093/molbev/msw054
Kutkienė L (2001) The species composition of European roe deer (Capreolus capreolus) Sarcocystis in Lithuania. Acta Zool Lituanica 11:97–101. doi:10.1080/13921657.2001.10512363
Kutkienė L, Prakas P, Sruoga A, Butkauskas D (2010) The mallard duck (Anas platyrhynchos) as intermediate host for Sarcocystis wobeseri sp. nov. from the barnacle goose (Branta leucopsis). Parasitol Res 107:879–888. doi:10.1007/s00436-010-1945-4
López C, Panadero R, Bravo A, Paz A, Sánchez-Andrade R, Díez- Banos P, Morrondo P (2003) Sarcocystis spp. infection in roe deer (Capreolus capreolus) from the north-west of Spain. Z Jagdwiss 49:211–218. doi:10.1007/bf02189739
Milne I, Wright F, Rowe G, Marshall DF, Husmeier D, McGuire G (2004) TOPALi: software for automatic identification of recombinant sequences within DNA multiple alignments. Bioinformatics 20:1806–1807. doi:10.1093/bioinformatics/bth155
Morrison DA, Bornstein S, Thebo P, Wernery U, Kinne J, Mattsson JG (2004) The current status of the small subunit rRNA phylogeny of the coccidian (Sporozoa). Int J Parasitol 34:501–514. doi:10.1016/j.ijpara.2003.11.006
Odening K, Stolte M, Walter G, Bockhardt I (1994) The European badger (Carnivora: Mustelidae) as intermediate host of further three Sarcocystis species (Sporozoa). Parasite 1:23–30. doi:10.1051/parasite/1994011023
Poli A, Mancianti F, Marconcini A, Nigro M, Colagreco R (1988) Prevalence, ultrastructure of the cyst wall and infectivity for the dog and cat of Sarcocystis sp. from fallow deer (Cervus dama). J Wildl Dis 24:97–104. doi:10.7589/0090-3558-24.1.97
Prakas P (2011) Diversity and ecology of Sarcocystis in Lithuanian game fauna. PhD thesis, Vilnius University, Vilnius, Lithuania
Prakas P, Butkauskas D, Rudaitytė E, Kutkienė L, Sruoga A, Pūraitė I (2016) Morphological and molecular characterization of Sarcocystis taeniata and Sarcocystis pilosa n. sp. from the sika deer (Cervus nippon) in Lithuania. Parasitol Res 115:3021–3032. doi:10.1007/s00436-016-5057-7
Rátz S (1909) Die Sarcosporidien und ihre in Ungarn vorkommenden Arten. Allattani Közlemények 8:1–37
Reissig EC, Moré G, Massone A, Uzal FA (2016) Sarcocystosis in wild red deer (Cervus elaphus) in Patagonia. Argentina Parasitol Res 115:1773–1778. doi:10.1007/s00436-016-4915-7
Santini S, Mancianti F, Nigro M, Poli A (1997) Ultrastructure of the cyst wall of Sarcocystis sp. in roe deer. J Wildl Dis 33:853–859. doi:10.7589/0090-3558-33.4.853
Schramlová J, Blažek K (1978) Ultrastruktur der Cystenwand der Sarkosporidien des Rehes (Capreolus capreolus L.). Z Parasitenkd 55:43–48. doi:10.1007/bf00383473
Sedlaczek J, Wesemeier HH (1995) On the diagnostics and nomenclature of Sarcocystis species (Sporozoa) in roe deer (Capreolus capreolus). Appl Parasitol 36:73–82
Speer CA, Dubey JP (1982) Sarcocystis wapiti sp. nov. from the North American wapiti (Cervus elaphus). Can J Zool 60:881–888. doi:10.1139/z82-120
Spickschen C, Pohlmeyer K (2002) Untersuchung zum Vorkommen von Sarkosporidien bei Reh-, Rot- und Muffelwild in zwei unterschiedlichen Naturräumen des Bundeslandes Niedersachsen. Z Jagdwiss 48:35–48. doi:10.1007/bf02285355
Tenter AM, Baverstock PR, Johnson AM (1992) Phylogenetic relationships of Sarcocystis species from sheep, goats, cattle and mice based on ribosomal RNA sequences. Int J Parasitol 22:503–513. doi:10.1016/0020-7519(92)90151-a
Wesemeier HH, Sedlaczek J (1995a) One known Sarcocystis species and one found for the first time in fallow deer (Dama dama). Appl Parasitol 36:299–302
Wesemeier HH, Sedlaczek J (1995b) One known Sarcocystis species and two found for the first time in red deer and wapiti (Cervus elaphus) in Europe. Appl Parasitol 36:245–251
Acknowledgments
This research was supported by the Open Access to research infrastructure of the Nature Research Centre under Lithuanian open access network initiative. The authors are grateful to Ms. S. Amšiejienė from the National Centre of Pathology (Vilnius, Lithuania) for her help in carrying out electron microscopy investigations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prakas, P., Rudaitytė, E., Butkauskas, D. et al. Sarcocystis entzerothi n. sp. from the European roe deer (Capreolus capreolus). Parasitol Res 116, 271–279 (2017). https://doi.org/10.1007/s00436-016-5288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5288-7