Abstract
Besnoitia besnoiti is an apicomplexan parasite and the causative agent of bovine besnoitiosis which is considered as a re-emergent disease in Europe. A cross-sectional serological study was conducted to determine the seroprevalence and to identify risk factors associated with B. besnoiti infection in 68 dairy herds (n = 806 cows) in Jordan during the period from January to June 2007 and the spring of 2014. Data regarding herd’s management was obtained by filling questionnaires through personal interviews with farmers. An indirect ELISA test was used to detect antibodies against B. besnoiti. Chi-square analysis and multivariable logistic regression model were used to identify risk factors associated with seropositivity to B. besnoiti. At the individual cow and herd level, the true prevalence of seropositive animals was 6 and 28.7 %, respectively. Cows between 2 and 6 years of age had significantly higher seroprevalence of B. besnoiti than other age groups. The highest seroprevalence of B. besnoiti was found in Zarqa and Irbid governorates. Multivariable logistic regression model identified that exchanging visits by farm workers to neighboring farms as a risk factor for seropositivity to B. besnoiti, while smaller herd size and twice a day farm cleaning using sweeping and water hosing were identified as protective factors. This is the first study that investigated the seroprevalence of B. besnoiti infection in dairy herds in Jordan. Further studies are warranted to explore the clinical manifestation of B. besnoiti infection as well as to identify the possible presence of other Besnoitia species and definitive hosts for the parasite.
Similar content being viewed by others
Introduction
Besnoitia besnoiti, the causative agent of bovine besnoitiosis, is an obligate intracellular apicomplexan parasite that is closely related to Toxoplasma gondii and Neospora caninum (Uzeda et al. 2014). Bovine besnoitiosis is highly prevalent in tropical and subtropical regions and has been reported from Africa (Penzhorn and Krecek 1997), Asia (Nobel et al. 1981), South America (Uzeda et al. 2014) and Europe (Alvarez-Garcia et al. 2013). It has been considered as a re-emergent disease in Europe by the European Food Safety Authority (EFSA 2010). Cattle and wild bovid (i.e., antelopes) act as the intermediate hosts for the parasite. The definitive host, which sheds oocysts after ingestion of infected tissues, remains unknown although a role for cats has been suggested in other Besnoitia species (Olias et al. 2011). Horizontal direct and indirect transmission, such as transport of subclinically infected cattle, seems to be responsible for the spread of the parasite not only between herds, but also among countries (Basso et al. 2011). Asexual and infective stage life cycle develops in cattle. In the acute phase of the disease, tachyzoites (endozoytes) invade endothelial cells of blood vessels resulting in vasculitis, hyperplasia, thrombosis, and necrosis of capillaries. Clinical signs associated with this phase include fever, anorexia, subcutaneous edemas, enlarged lymph nodes, nasal and ocular discharge, weight loss, abortion, and infertility of bulls due to orchitis. The subsequent, chronic phase of the disease is characterized by the formation of large numbers of parasitic cysts containing bradyzoites (cystozoites) which can persist for several years in various organs of the host (Cortes et al. 2014). Skin thickening, hardening, wrinkling, and hair loss and presence of visible, pathognomonic thick-walled tissue cysts in the scleral conjunctivae and genital mucosa are manifestation of the chronic stage of besnoitiosis (Basso et al. 2013; Gollnick et al. 2015). Fortunately, the disease usually occurs sporadically, most infections are subclinical and the mortality rate is less than 10 % (Cortes et al. 2005; Olias et al. 2011). However, significant financial losses in cattle herds occur due to high morbidity (80 %), decreased milk and meat production, reduced value of the hides for leather production, impairment of reproductive performance and culling of infected animals (Jacquiet et al. 2010).
In order to avoid the entry of infected animals into B. besnoiti-free herds and to better understand the epidemiology of besnoitiosis, diagnosis of B. besnoiti infection becomes crucial. Traditional methods of diagnosing bovine besnoitiosis include detection of the macroscopic cysts in the scleral conjunctiva, or the observation of clinical signs such as skin lesions. Serological tests such as indirect fluorescent antibody test (IFAT), enzyme-linked immunosorbent assays (ELISA), modified agglutination test (MAT), and Western blot are used for determination of antibody levels against B. besnoiti in sera of infected animals including asymptomatic carriers. Histopathological examination and detection of specific DNA using the polymerase chain reaction (PCR) of skin biopsies are used to confirm the diagnosis (Fernandez-Garcia et al. 2010).
The investigation of herd seroprevalence status is an initial step to evaluate exposure of cattle population to B. besnoiti. In addition, determination of possible risk factors for herds to acquire B. besnoiti infection is important for the implementation of control measures to prevent disease transmission and to minimize adverse effects of the infection on herd health and productivity. To the best of the authors’ knowledge, there is no previous report about besnoitiosis or epidemiological study of B. besnoiti in dairy herds in Jordan. Therefore, the aims of this study were to estimate the seroprevalence of B. besnoiti infection at the individual and herd levels and to identify potential risk factors associated with seropositivity to the parasite.
Materials and methods
Herd management
According to the report provided by the Jordanian department of statistics (DOS 2013), there were about 69,739 dairy cows in Jordan. Ninety-nine percent of cows were Holstein–Friesian while the rest were of the Baladi and Shami breeds. All cows sampled in this study were Holstein–Friesian raised in open stalls with fence line separation between heifers and adult cows. The cows were milked twice daily and most herds were fed total mixed rations and grass (alfalfa, silage, and clover) when available. Most cows were vaccinated against foot and mouth disease, enterotoxemia, and anthrax. No other vaccines were used. All herds used anthelmintic medications on a regular basis. Artificial insemination was usually used for most females but use of bulls for natural breeding was also part of some farms’ breeding management. Most male calves were sold either soon after birth or weaning.
Study design
Part of the data and samples used in this study were collected from a previous cross-sectional seroprevalence study conducted between January and June 2007 (Talafha et al. 2009). More data and samples were collected in the spring of 2014. The required sample size was determined with 98 % confidence interval, percent of accepted error of ±4 and expected prevalence of 10 % using C-survey software 2.0 (UCLA, Los Angeles, CA, USA). The resulted sample size was adjusted to the cattle population in Jordan. Accordingly, 806 cows were sampled from 68 herds from 10 out of 12 Jordanian governorates (Amman, Balqa, Zarqa, Madaba, Irbid, Mafraq, Jarash, Ajloun, Karak, and Tafielah). Maan and Aqaba governorates were not included in the study due to the very small population of cattle in these governorates (182 and 3 cows, respectively). Number of cows to sample from each governorate depended on the density of cows in that governorate. The records of the Jordanian Ministry of Agriculture were used to randomly select the cattle herds. A table of random digits was used to randomly select cows from each herd. Only cows >12 months old were bled since progression of infection with B. besnoiti proceeds slowly and gender has not been found to be a risk factor for the infection (Alvarez-Garcia et al. 2013). In addition, the number of bulls available in the dairy farms was very limited. Cows were in apparent healthy status and clinical signs of besnoitiosis, particularly the presence of macroscopic cysts in ocular sclera and vulva, were not searched from sampled cow. Herds were stratified into three herd size strata: small (<50 cows), medium (50–150 cows), and large (>150 cows).
During the visit to each farm, a pre-tested structured questionnaire was served on the farm owner or manager in order to gather data about potential risk factors associated with B. besnoiti infection (Table 1).
The general climate of Jordan
Jordan is located about 80 km to the east of the Mediterranean Sea between 31°N and 36°E coordinates with an area of 88,778 km2. The climate of Jordan is predominantly of the Mediterranean type. It is characterized by hot dry summer with average temperature in the mid 30 °C and relatively cold and wet winter with temperature averaging around 13 °C. Jordan is divided into three main climatic regions. The first one is the Jordan Valley. It extends for about 400 km from North to South with a width varies from 10 km in the North to 30 km in the South. It is between 170 and 400 m below Mean Sea Level (MSL). The second is the highlands region which is east of the Jordan Valley. This mountain range is about 1,150 m above MSL in the northern parts and about 1,500 m above MSL in the southern parts of the country. About 88 % of human settlement of Jordan lives in this region. The last is the Badia and desert region which is located to the East of this mountain range. It is a semi desert plateau that extends to cover about 80 % of the total area of the country. Most of Jordan (90 %) is arid and semi-arid areas that are characterized by remarkable rainfall variation with total annual rainfall averages less than 200 mm (JOMETEO 2012; RJGC 2012). Administratively, Jordan is divided into 12 provinces called governorates.
Dairy production systems in Jordan
There are two dairy production systems in Jordan, the intensive and small-scale production systems (Alqaisi et al. 2009). The former is characterized by herds where the number of cows per herd exceeds 10 dairy cows, stanchion barns are used, and the average milk production is about 6,000 kg/cow/year. This system is very common in the eastern semi-arid area of the country. The latter exists in different parts of the country, but mainly in the Jordan Valley and the northern highlands regions. Small and fluctuating herd size (usually less than 10 dairy cows per herd), poor feeding resources, lower milk production (about 4,500 kg/cow/year), less health and management practices and housing of cows in small traditional brick barns are characteristics of this system.
Sample collection
Blood samples (about 10 ml) were obtained from all cows by puncture of the coccygeal vein using sterile disposable needles and tubes without anticoagulant (Occidem Biotech®, Middlesex, UK). Blood samples were kept on ice until and during transportation to the laboratory. Sera were separated by centrifugation at 5,000×g for 10 min and dispensed into plastic vials with screw caps. Sera were properly identified and stored frozen at −20 °C until analysis.
Laboratory analysis
To detect antibodies against B. besnoiti, serum samples were analyzed by a commercially available indirect enzyme-linked immunosorbant assay (ELISA) kit (The PrioCHECK® Besnoitia besnoiti Antibody 2.0 Test Kit®, Prionics AG, Schlieren-Zurich, Switzerland). The test was performed according to the manufacturer’s instructions using a cut-off threshold of 23 percent positivity. Both positive and negative control sera were included in each assay. The assay has a sensitivity of 97.8 % and a specificity of 98.1 %.
Statistical analysis
The apparent individual and herd prevalence were calculated as: (number of antibody positive animals (or herds)/number of sampled animals (or herds) × 100. A herd was considered positive when at least one seropositive cow was found in the herd. True prevalence was calculated by adjusting the apparent prevalence to the test sensitivity and specificity. The following equation was used for calculating the true prevalence: TP = (AP + Sp − 1)/(Se + Sp − 1) where TP is the true prevalence, AP is the apparent prevalence, Se is the test sensitivity, and Sp is the test specificity (Rogan and Gladen 1978). Collected data were cleaned and coded into a specially designed SPSS spread sheet. Univariable analysis was carried out using Chi-square test. Variables with P value ≤ 0.05 were offered to multivariable logistic regression analysis. Regions from where samples were collected were analyzed using step-wise LSD analysis. All analyses were performed using the IBM statistical software package SPSS, version 20.
Results
Testing 806 serum samples for B. besnoiti antibodies, the ELISA identified 7.7 % (62/806) of individual cattle sera were seropositive. At the herd level, 29.4 % (20/68) of cattle herds had at least one cow presenting antibodies to B. besnoiti. When adjusted to the test sensitivity and specificity the true individual and herd seroprevalences were 6 and 28.7 %, respectively. Cows between 2–4 and 4–6 years of age had significantly higher (χ 2; P < 0.05) seroprevalence of B. besnoiti than other age groups (Table 2). Zarqa and Irbid governorates had significantly higher (χ 2; P < 0.05) seroprevalence of B. besnoiti than the other Jordanian governorates (Amman, Balqa, Madaba, Mafrak, Jarash, Ajloun, Karak, and Tafielah).
The Chi-square univariable analysis revealed five variables (Table 1) with P ≤ 0.05 that were further offered to the logistic analysis. Farm workers exchange visits of neighboring farms (within few kilometers) more than two to three times a week (OR = 5.01; 95 % CI 1.08, 23.22) was identified as a risk factor for seropositivity to B. besnoiti, while smaller herd size (OR = 0.076; 95 % CI 0.008, 0.757) and frequency of cleaning (OR = 0.086; 95 % CI 0.01; 0.73) were identified as protective factors (Table 3).
Discussion
To the authors’ knowledge, this is the first study that documents the seroprevalence of B. besnoiti in Jordan’s dairy herds. In the present study, B. besnoiti true seroprevalence for individual cows and herds were 6 and 28.7 %, respectively. Many studies were conducted to determine the seroprevalence of B. besnoiti infection in cattle herds. Ashmawy and Abu-Akkada (2014) reported that the percentage of B. besnoiti-positive dairy cows in five provinces of Egypt was 17.1 % (37/216). In Israel, a seroprevalence rate of 10 % for dairy cattle was reported when 1,700 animals were tested (Goldman and Pipano 1983). In Italy, Rinaldi et al. (2013) reported a prevalence of 44.1 % (233/528) and 83 % (73/88) in animals and herds, respectively. A study conducted in Australia identified 18.4 % of 869 individual cattle were positive by the PrioCHECK® Besnoitia Ab 2.0 ELISA test (Nasir et al. 2012). The seroprevalence of B. besnoiti infection in cattle varies considerably among studies. These variations might be due to differences in animal breed (beef versus dairy), the serologic tests and their cut-off thresholds used, study design, cattle management systems, different levels of contact between infected and non-infected cattle, and exposure to reservoirs of infection and insect vectors.
The seroprevalence of B. besnoiti in cattle in Zarqa and Irbid governorates was significantly higher than that found in other Jordanian governorates. In these two governorates, it was noticed that the dairy farms were built on a smaller size farm land and the distance to neighboring farms is small. Significant associations between seroprevalence and herd location were observed. A study in Portugal reported a variation between 0.7 and 72.4 % in the overall within-herd prevalence and seropositive herds were detected for the first time in the region Centro and in the northeast of the country (Waap et al. 2014). The effect of region on the seroprevalence of B. besnoiti was documented by other studies. Alvarez-Garcia et al. (2014b) reported that seropositive beef cattle were located in mountainous regions where the disease is endemic in the province of Huesca (Aragon, Spain). In Italy, significant variation was found in the number of seropositive animals among the four studied regions in the southern part of the country (Gazzonis et al. 2014). Recent epidemiological data in Europe confirmed the increased prevalence and geographical expansion of this disease to previously free neighboring regions and countries (EFSA 2010). Variations in the seroprevalence of B. besnoiti in different regions might be due to high variety of cattle management and production systems, differences in landscape, rainfall averages and climate, time of the year the study was performed and availability of proper habitat for the insect vectors to spread the infection (Gazzonis et al. 2014).
In the present study cows between 2 and 6 years of age had significantly higher seroprevalence of B. besnoiti than other age groups. An increase in seropositivity with age has been reported. Fernandez-Garcia et al. (2010) and Alvarez-Garcia et al. (2014a) found an increase in seroprevalence as cows get older. However, the lack of significant increase in seroprevalence in cows older than 6 years of age in this study might be due to the small sample size and the limited number of old cows available in the dairy farms. Results from these studies support the hypotheses that horizontal transmission is responsible for the parasite dissemination in a herd and older cows have a greater chance of becoming infected due to longer period of exposure. This finding is consistent with previous studies (EFSA 2010).
Three variables were significantly (P < 0.05) associated with seropositivity to B. besnoiti in the multivariable logistic regression analyses (Tables 1 and 3). The seroprevalence of B. besnoiti was significantly higher in farms where workers exchange visits with neighboring farms workers than that of farms where such visits were not allowed (35 and 21 %, respectively). It is a common practice in Jordan’s dairy herds where workers of neighboring farms help each other’s in routine dairy work with very limited attention to biosecurity. Although the life cycle of B. besnoiti is not fully understood, transmission is thought to occur mainly by direct contact between infected and non-infected cattle. Biting insects, communal pastures, direct contact throughout natural mating, wounds, and lacerations and animal trade play a major role in the dissemination of the parasite (Alvarez-Garcia et al. 2014b). Other possible modes of transmission may include workers clothing, sharing contaminated farm equipments, injectables and biologicals, airborne transmission and exposure of cattle to sporulated oocysts in the environment or to feed source infected or contaminated with tachyzoites or bradyzoites of B. besnoiti to which the definitive host has access (Tenter and Johnson 1997). Vertical transmission has not been proven since chronically infected cows may become pregnant and successfully give birth to healthy calves that develop normally (Alvarez-Garcia et al. 2014b). In addition, the semen of chronically infected bulls is very unlikely source of B. besnoiti infection (Esteban-Gil et al. 2014). Exposure to still unidentified risk factors may play a role in the horizontal transmission of the parasite.
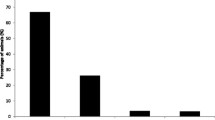
In this study, the influence of herd size on B. besnoiti distribution showed that cows in smaller herd’s size are less likely to become infected with B. besnoiti than those in larger herds (23, 31, and 80 %, for small, medium, and large size herds, respectively). In agreement with our study, Waap et al. (2014) reported that in herds where number of cows was 5–83, the proportion of positive animals (1.1 %) was significantly lower compared with 5.1, 3.6, and 2.6 % for herds with number of cows was 84–167, 168–307, and 308–4,328, respectively.
Another factor that was found to be protective against infection with B. besnoiti was the frequency of farm cleaning (25, 6, and 44 %) for once a day, twice a day, and once a month cleaning, respectively. Alvarez-Garcia et al. (2013) reported that B. besnoiti sporulated oocysts are excreted in the feces and are resistant to the environment; similar to the oocysts of T. gondii and N. caninum. Since the spread of B. besnoiti is linked to management practices that favor its dissemination, it becomes important to take management measures coupled to diagnosis in order to control bovine besnoitiosis. Avoiding direct and indirect transmission, a decrease in disease prevalence may be achieved (Alvarez-Garcia et al. 2013). Frequent farm cleaning by sweeping and water hosing, and use of disinfectants may result in reduction of the number of sporulated oocysts in the environment and therefore decreases the likelihood of cattle acquiring the infection. The best type of disinfectant to be used against B. besnoiti oocysts still needs to be determined.
Conclusions
This is the first epidemiological study to report the individual and herd prevalence of B. besnoiti infection and risk factors associated with the parasite in dairy herds in Jordan. Minimizing farm workers visiting other farms and keeping the cow environment as clean as possible are important measures to minimize the risk of transmission of B. besnoiti among and within herds and to improve the herd health and productivity. More studies are needed to confirm the presence of B. besnoiti and possibly other Besnoitia species in Jordan’s cattle and to develop control programs to prevent transmission of the parasite.
References
Alqaisi O, Ndambi OA, Hemme T (2009) Development of milk production and the dairy industry in Jordan. Livestock Research for Rural Development. Volume 21, Article #107. Retrieved March 2, 2015, from http://www.lrrd.org/lrrd21/7/alqa21107.htm
Alvarez-Garcia G, Frey CF, Mora LM, Schares G (2013) A century of bovine besnoitiosis: an unknown disease re-emerging in Europe. Trends Parasitol 29(8):407–415
Alvarez-Garcia G, Fernandez-Garcia A, Gutierrez-Exposito D, Quiteria JA, Aguado-Martinez A, Ortega-Mora LM (2014a) Seroprevalence of Besnoitia besnoiti infection and associated risk factors in cattle from an endemic region in Western Europe (Navarra, Spain). Vet J 200:328–331
Alvarez-Garcia G, Garcia-Lunar P, Gutierrez-Exposito D, Shkap V, Ortega-Mora LM (2014b) Dynamics of Besnoitia besnoiti infection in cattle. Parasitology 141(11):1419–1435
Ashmawy KI, Abu-Akkada SS (2014) Evidence for bovine besnoitiosis in Egypt-first serosurvey of Besnoitia besnoiti in cattle and water buffalo (Bubalus bubalis) in Egypt. Trop Anim Health Prod 46(3):519–522
Basso W, Schares G, Gollnick NS, Rutten M, Deplazes P (2011) Exploring the life cycle of Besnoitia besnoiti—experimental infection of putative definitive and intermediate host species. Vet Parasitol 178(3–4):223–234
Basso W, Lesser M, Grimm F, Hilbe M, Sydler T, Trosch L, Ochs H, Braun U, Deplazes P (2013) Bovine besnoitiosis in Switzerland: imported cases and local transmission. Vet Parasitol 198(3–4):265–273
Cortes H, Leitao A, Vidal R, Vila-Vicosa MJ, Ferreira ML, Caeiro V, Hjerpe CA (2005) Besnoitiosis in bulls in Portugal. Vet Rec 157(9):262–264
Cortes H, Leitao A, Gottstein B, Hemphill A (2014) A review on bovine besnoitiosis: a disease with economic impact in herd health management, caused by Besnoitia besnoiti (Franco and Borges). Parasitology: 1–12
Department of Statistics (2013) The Hashemite Kingdom of Jordan. Agricultural surveys in 2013, Jordan. http://www.dos.gov.jo/dos_home_e/main/ Accessed March 3, 2015
EFSA European Food Safety Authority (2010) Bovine besnoitiosis: an emerging disease in Europe. Scientific statement on bovine besnoitiosis, Question No EFSA-Q-2009-00879, EFSA Journal 8, 1499. http://www.efsa.europa.eu/en/efsajournal/pub/1499.htm. Adopted 28 January 2010.
Esteban-Gil A, Grisez C, Prevot F, Florentin S, Decaudin A, Picard-Hagen N, Berthelot X, Ronsin P, Alzieu JP, Marois M, Corboz N, Peglion M, Vilardell C, Lienard E, Bouhsira E, Castillo JA, Franc M, Jacquiet P (2014) No detection of Besnoitia besnoiti DNA in the semen of chronically infected bulls. Parasitol Res 113(6):2355–2362
Fernandez-Garcia A, Alvarez-Garcia G, Risco-Castillo V, Aguado-Martinez A, Marcen JM, Rojo-Montejo S, Castillo JA, Ortega-Mora LM (2010) Development and use of an indirect ELISA in an outbreak of bovine besnoitiosis in Spain. Vet Rec 166(26):818–822
Gazzonis AL, Garcia G, Zanzani SA, Garippa G, Rossi L, Maggiora M, Dini V, Invernizzi A, Luini M, Tranquillo VM, Mora L, Manfredi M (2014) Besnoitia besnoiti among cattle in insular and northwestern Italy: endemic infection or isolated outbreaks? Parasit Vectors 7(1):585–593
Goldman M, Pipano E (1983) Serological studies on bovine besnoitiosis in Israel. Trop Animal Health Prod 15(1):32–38
Gollnick NS, Scharr JC, Schares G, Langenmayer MC (2015) Natural Besnoitia besnoiti infections in cattle: chronology of disease progression. BMC Vet Res 11(1):35
Jacquiet P, Lienard E, Franc M (2010) Bovine besnoitiosis: epidemiological and clinical aspects. Vet Parasitol 174(1–2):30–36
Jordan Meteorological Department (2012). Meteorological Department. http://www.jometeo.gov.jo. Accessed 3 June 2012
Nasir A, Lanyon SR, Schares G, Anderson ML, Reichel MP (2012) Sero-prevalence of Neospora caninum and Besnoitia besnoiti in South Australian beef and dairy cattle. Vet Parasitol 186:480–485
Nobel T, Klopfer U, Perl S, Nyska A, Neumann M, Brenner G (1981) Histopathology of genital besnoitiosis of cows in Israel. Vet Parasitol 8(4):271–276
Olias P, Schade B, Mehlhorn H (2011) Molecular pathology, taxonomy and epidemiology of Besnoitia species (Protozoa: Sarcocystidae). Infect Genet Evol 11:1564–1576
Penzhorn BL, Krecek RC (1997) Veterinary parasitology in SouthAfrica: some highlights of the past 100 years. Vet Parasitol 71:69–76
Rinaldi L, Maurelli MP, Musella V, Bosco A, Cortes H, Cringoli G (2013) First cross-sectional serological survey on Besnoitia besnoiti in cattle in Italy. Parasitol Res 112(4):1805–1807
Rogan WJ, Gladen B (1978) Estimating prevalence from the results of a screening test. Am J Epidemiol 107(1):71–76
Royal Jordanian Geographic Center (2012). http://www.rjgc.gov.jo Accessed 3 June, 2012
Talafha AQ, Hirche SM, Ababneh MM, Al-Majali AM, Ababneh MM (2009) Prevalence and risk factors associated with bovine viral diarrhea virus infection in dairy herds in Jordan. Trop Animal Health Prod 41:499–506
Tenter AM, Johnson AM (1997) Phylogeny of the tissue cyst forming coccidian. Adv Parasitol 39:69–139
Uzeda RS, Andrade MR, Corbellini LG, Antonello AM, Vogel FS, Gondim LF (2014) Frequency of antibodies against Besnoitia besnoiti in Brazilian cattle. Vet Parasitol 199(3–4):242–246
Waap H, Nunes T, Cortes H, Leitão A, Vaz Y (2014) Prevalence and geographic distribution of Besnoitia besnoiti infection in cattle herds in Portugal. Parasitol Res 113(10):3703–3711
Acknowledgements
The authors would like to thank dairy farms owners and managers for kindly providing information and blood samples. The work was supported by a research grant from Deanship of Research, Jordan University of Science and Technology, Irbid, Jordan.
Conflict of interest
The authors declare that there is no potential financial or personal conflict of interest with other people or organizations regarding the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talafha, A.Q., Al-Majali, A.M., Ababneh, M.M. et al. Epidemiologic study on Besnoitia besnoiti infection in dairy herds in Jordan. Parasitol Res 114, 2491–2497 (2015). https://doi.org/10.1007/s00436-015-4448-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4448-5