Abstract
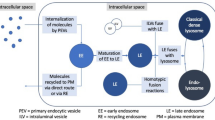
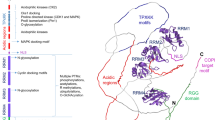
Small ubiquitin-like modifier (SUMO) conjugation of proteins occurs through a concert action of enzymes using a similar ubiquitination mechanism. After a C-terminal peptide is cleaved from the SUMO precursor by a protease to reveal a di-glycine motif, SUMO is activated by an E1 enzyme (Aos1/Uba2) and conjugated to target proteins by the sole E2 enzyme (Ubc9) guided to the appropriate substrates by the SUMO E3 ligase. Previous reports from our group showed that Schistosoma mansoni has two paralogs of SUMO: one E2 conjugation Ubc9 and two SUMO-specific proteases (SENPs). The differential gene expression profile observed for SUMO pathway genes throughout the S. mansoni life cycle attests for the distinct patterns of SUMO conjugates observed during parasite development particularly during the cercariae to schistosomula transition. To continue this investigation, we here analysed the repertoire of SUMO E3 ligases and their expression profiles during cercariae/schistosomula transition. In silico analysis through S. mansoni databases showed two conserved SUMO E3 ligases: protein inhibitor of activated STAT (PIAS) and Ran-binding protein 2 (RanBP2). Furthermore, expression levels of the SUMO E3 ligases were measured by qRT-PCR using total RNA from cercariae, adult worms and mechanically transformed schistosomula. Our data showed an up-regulation of expression in lung schistosomula and adult worm stages. In conclusion, the differential expression of SmPIAS and SmRanBP2 during schistosomula development was similar to the expression levels of all genes related to SUMO conjugation, thereby suggesting that the control of protein activity, localisation or stability during cercariae to schistosomula transition is SUMO-dependent.

Similar content being viewed by others
References
Azuma Y, Dasso M (2002) A new clue at the nuclear pore: RanBP2 is an E3 enzyme for SUMO1. Dev Cell 2:130–131
Basch P, DiConza J (1977) In vitro development of Schistosoma mansoni cercariae. J Parasitol 63:245–249
Cabral FJ, Pereira OS Jr, Silva CS, Guerra-Sa R, Rodrigues V (2008) Schistosoma mansoni encodes SMT3B and SMT3C molecules responsible for post-translational modification of cellular proteins. Parasitol Int 57:172–178
Duval D, Duval G, Kedinger C, Poch O, Boeuf H (2003) The ‘PINIT’ motif, of a newly identified conserved domain of the PIAS protein family, is essential for nuclear retention of PIAS3 L. FEBS Lett 554(1–2):111–118
Fang S, Lorick KL, Jensen JP, Weissman AM (2003) RING finger ubiquitin protein ligases: implications for tumorigenesis, metastasis and for molecular targets in cancer. Semin Cancer Biol 13:5–14
Fried H, Kutay U (2003) Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci 60:1659–1688
Gareau JR, Lima CD (2010) The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol 11:861–871
Geiss-Friedlander R, Melchior F (2007) Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8:947–956
Gill G (2005) SUMO changes Sox for developmental diversity. Mol Cell 20:495–496
Gregoire S, Yang XJ (2005) Association with class IIa histone deacetylases upregulates the sumoylation of MEF2 transcription factors. Mol Cell Biol 25:2273–2287
Hammer E, Heilbronn R, Weger S (2007) The E3 ligase Topors induces the accumulation of polysumoylated forms of DNA topoisomerase I in vitro and in vivo. FEBS Lett 581:5418–5424
Han ZG, Brindley PJ, Wang SY, Chen Z (2009) Schistosoma genomics: new perspectives on schistosome biology and host-parasite interaction. Annu Rev Genomics Hum Genet 10:211–240
Harrop R, Wilson RA (1993) Protein synthesis and release by cultured schistosomula of Schistosoma mansoni. Parasitology 107:265–274
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Hochstrasser M (2001) SP-RING for SUMO: new functions bloom for a ubiquitin-like protein. Cell 107:5–8
Hogarth C, Itman C, Jans DA, Loveland KL (2005) Regulated nucleocytoplasmic transport in spermatogenesis: a driver of cellular differentiation? Bioessays 27:1011–1025
Jackson PK (2001) New RING for SUMO: wrestling transcriptional responses into nuclear bodies with PIAS family E3 SUMO ligases. Genes Dev 15:3053–3058
Jackson PK, Eldridge AG (2002) The SCF ubiquitin ligase: an extended look. Mol Cell 9:923–925
Joazeiro CA, Weissman AM (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell 102:549–552
Johnson ES, Gupta AA (2001) An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106:735–744
Kagey MH, Melhuish TA, Wotton D (2003) The polycomb protein Pc2 is a SUMO E3. Cell 113:127–137
Kahyo T, Nishida T, Yasuda H (2001) Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol Cell 8:713–718
Kotaja N, Karvonen U, Janne OA, Palvimo JJ (2002) PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol Cell Biol 22:5222–5234
Lima CD (2002) Bridging the gap between SCF and ubiquitin transfer. Structure 10:741–742
Liu S, Cai P, Hou N, Piao X, Wang H, Hung T, Chen Q (2012) Genome-wide identification and characterization of a panel of house-keeping genes in Schistosoma japonicum. Mol Biochem Parasitol 182:75–82
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Melchior F (2000) SUMO-nonclassical ubiquitin. Annu Rev Cell Dev Biol 16:591–626
Palvimo JJ (2007) PIAS proteins as regulators of small ubiquitin-related modifier (SUMO) modifications and transcription. Biochem Soc Trans 35:1405–1408
Pereira RV, Cabral FJ, Gomes MS, Baba EH, Jannotti-Passos LK, Carvalho O, Rodrigues V, Afonso RJ, Castro-Borges W, Guerra-Sá R (2011) Molecular characterization of SUMO E2 conjugation enzyme: differential expression profile in Schistosoma mansoni. Parasitol Res 109:1537–1546
Pereira RV, Cabral FJ, de Souza Gomes M, Jannotti-Passos LK, Castro-Borges W, Guerra-Sá R (2012) Transcriptional profile and structural conservation of SUMO-specific proteases in Schistosoma mansoni. J Parasitol Res 480824:1–7
Pichler A, Gast A, Seeler JS, Dejean A, Melchior F (2002) The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108:109–120
Pichler A, Knipscheer P, Saitoh H, Sixma TK, Melchior F (2004) The RanBP2 SUMO E3 ligase is neither HECT- nor RING-type. Nat Struct Mol Biol 11:984–991
Pickart CM (2001) Ubiquitin enters the new millennium. Mol Cell 8:499–504
Ross S, Best JL, Zon LI, Gill G (2002) SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol Cell 10:831–842
Rytinki MM, Kaikkonen S, Pehkonen P, Ja¨A¨Skela¨Inen T, Palvimo JJ (2009) PIAS proteins: pleiotropic interactors associated with SUMO. Cell Mol Life Sci 66:3029–3041
Sachdev S, Bruhn L, Sieber H, Pichler A, Melchior F, Grosschedl R (2001) PIASy, a nuclear matrix-associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev 15:3088–3103
Sapetschnig A, Rischitor G, Braun H, Doll A, Schergaut M, Melchior F, Suske G (2002) Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J 21:5206–5215
Seeler JS, Dejean A (2003) Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol 4:690–699
Takahashi Y, Kahyo T, Toh EA, Yasuda H, Kikuchi Y (2001) Yeast Ull1/Siz1 is a novel SUMO1/Smt3 ligase for septin components and functions as an adaptor between conjugating enzyme and substrates. J Biol Chem 276:48973–48977
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Verger A, Perdomo J, Crossley M (2003) Modification with SUMO. A role in transcriptional regulation. EMBO Rep 4:137–142
Yokoyama N, Hayashi N, Seki T, Panté N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U, Fukui M, Nishimoto T (1995) A giant nucleopore protein that binds Ran/TC4. Nature 376:184–188
Acknowledgments
The authors would like to thank the following transcriptome initiatives: the São Paulo Transcriptome Consortium, the Minas Gerais Genome Network and the Wellcome Trust Genome Initiative (UK). This work was supported by the following Brazilian research agencies: FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, CBB 0558/09), NuBio UFOP (Núcleo de Bioinformática da Universidade Federal de Ouro Preto) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Domain of SmPIAS. PIAS is characterised by an essential RING-like domain with similarities to the RING finger of ubiquitin E3 ligases. (JPEG 55 kb)
Fig. S2
Domain of SmRanBP2. RanBP2 is a component of the nuclear pore complex. This protein has domains that can bind to RanGTP and RanGDP, as well as repeats for nuclear transport receptor binding and a cyclophilin homology region but has no obvious similarity to other E3 ligases. (JPEG 108 kb)
Rights and permissions
About this article
Cite this article
Pereira, R.V., de S. Gomes, M., Cabral, F.J. et al. Up-regulation of SUMO E3 ligases during lung schistosomula and adult worm stages. Parasitol Res 113, 2019–2025 (2014). https://doi.org/10.1007/s00436-014-3841-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3841-9