Abstract
Calceoli are putative sensory organs which are known in limnic, marine, and subterranean amphipods for almost 200 years. Despite an otherwise comprehensive understanding of the sensory organs of crustaceans, we still have not unambiguously understood their function. Since calceoli are mainly found on the antennae of male animals, previous studies suggested a chemosensory function mainly related to reproduction. Here, we use a combination of light and electron microscopic techniques to examine the calceoli of Gammarus locusta (Linnaeus 1758) and Oediceroides calmani (Walker 1906), to provide an overview over these structures, and in addition reveal nervous tissue in close proximity to the calceoli. The calceoli of both species are cuticular structures and consist of proximal and distal elements, a stalk and a receptacle that connects both regions. The two studied calceoli differ in the structure of their proximal and distal element, as well as in their receptacle. This study provides new insight into the functional morphology of the antenna and calceolus. Histological sections through the antennae and the calceoli indicate that the calceoli might possess a mechanosensory function.
Similar content being viewed by others
Introduction
Calceoli are structures associated with the first antennules and the second pair of antennae and occur in about 10% of gammaridean amphipods (Hurley 1980). They appear in various families, e.g., Gammaridae, Oedicerotidae, Lysianassidae or Eusiridae as well as in amphipods from various aquatic habitats in all depth zones without an obvious pattern of distribution. Although the first calceolus was discovered almost 200 years ago, their exact function is still unknown. The first discovery of calceoli is attributed to Milne-Edwards (1830), who described these structures as “une petite copule membraneuse” (a small membranous copula). The Latin name relates to their shoe-shaped form (Latin calceolus: little boot). Indeed, several studies investigating the overall structure of this putative sensory organ have revealed its general structure: each calceolus consists of a concave proximal element and a distal element, which is covered with transversally arranged lamellae. Both elements are cuticular formations and supported by a receptacle, which is connected to the antenna by a stalk (Lincoln and Hurley 1981). Although every calceolus consists of a proximal and distal element, as well as a receptacle and a stalk, the appearance of these elements differs between individual species. Depending on the species (see more details below), calceoli are between 20 and 300 µm long and may occur individually or in groups on each antennal segment where they are arranged in rows along the antenna’s medial axis. The calceoli occur on the dorsomedial side of the first antennules and on the ventromedial side of the second antennae (Lincoln and Hurley 1981). Characteristically, calceoli are found only on the flagellum of the second antennae of males, such as in the majority of Gammarus species. However, there are species in which females also possess calceoli, such as in the family Eusiridae (Hurley 1980). In addition to occurring on the flagellum of the second antennae, calceoli may also occur on the peduncle of the first antennules in some species (Lincoln and Hurley 1981).
Lincoln and Hurley (1981) defined nine structural types based on the characterizing amphipod family: (1) gammarid, (2) bathyporeid, (3) lysianassid, (4) pontogeneiid, (5) eusirid, (6) gammarellid, (7) oedicerotid, (8) phoxocephalid, and (9) crangonycid. In their study, scanning electron microscopy (SEM) images of more than 60 different species from 40 genera were analysed for this classification and categorization, providing an overview of almost all calceoli-bearing families.
Their study showed that the occurrence of calceoli does not appear to be linked to the mode of life, habitat, or systematic affiliation (Lincoln and Hurley 1981). Moreover, no relationship has yet been established between species possessing calceoli and species in which they are absent. Even within a generally calceoli-bearing family, species without calceoli occur (Lincoln and Hurley 1981). The most recent described example is the genus Jesogammarus, in which all members of the genus possess calceoli except for the species J. acalceoli Tomikawa and Kimura 2021. Since the genus’ common ancestor possessed calceoli as well, it seems that calceoli were lost secondarily (Tomikawa and Kimura 2021). To complicate things even further, calceoli also do not appear in all populations of the same species (Cole 1970; Croker and Gable 1977). Since calceoli mostly appear on adult amphipods it has been suggested that the possession of calceoli is connected to maturity (see discussion). Amphipods can have multiple generations within a year, as observed in G. locusta (Costa and Costa 1999). Mekhanikova (2021) suggested that some populations do not have calceoli, because the males are in different moult stages. Moreover, calceoli-bearing species have no ecological or biological similarities (Lincoln and Hurley 1981): calceoli have been reported in predators, scavengers, filter feeders, and herbivores. Calceoli-bearing species furthermore colonize a variety of habitats, as they occur in marine as well as in brackish or freshwater environments, and have been found in the deep sea, shallow waters, polar and tropical regions (Lincoln and Hurley 1981). This broad and scattered distribution amongst amphipod species and high degree of inter- and intraspecies variation is one of the reasons that no compelling idea on their function has yet been suggested despite the many anatomical studies that have been performed to this date (Hurley 1980; Lincoln and Hurley 1981).
Their position mainly on the second pair of antennae and the main occurrence on males strongly suggested a sexual function of the calceoli (Bellan-Santini 2015). Mating occurs in all Amphipoda shortly after the female moults and is often preceded by either the so-called “precopula” or “mate guarding”: in precopula, the male grasps the female with its gnathopods and carries it along for some time. Mate guarding means that the male remains near the female until the moment of mating (Bellan-Santini 2015).
Dahl et al. (1970) were the first to attempt to reveal the function of calceoli experimentally. This study provided the basis for the assumption that the calceoli have a chemosensory function and can sense pheromones in the water. In the reported experiment, Gammarus duebeni (Lilljeborg 1852) females were fed trout that had previously been injected with radiolabelled chemicals, which were subsequently incorporated in the female organism. As a result, any pheromones produced by these females, that were absorbed by the males, should be localized in their antennae. The authors surmised that if the calceoli where chemosensory organs, they could be identified by microscopic autoradiography of male antennae. They indeed reported that the calceoli were the site of pheromone uptake. However, later studies, such as those by Lincoln and Hurley (1981), casted doubt on their conclusions due to the limited resolution of microscopic autoradiography, which does not allow a very accurate localization. The latter authors argued that the radioactively labelled substances were probably taken up by the neighbouring setae.
Hartnoll and Smith (1980), found no evidence that distance or contact pheromones play a role in the choice of a sexually mature mating partner during “mate assessment” in Gammarus duebeni (Lilljeborg 1852); a calceoli-bearing species in their experiments. Mating behaviour of calceoli-bearing amphipods was further studied by Read and Williams (1990) and Dunn (1998). The results showed that removing the antennae bearing calceoli had no effect on the ability of males to establish contact with a sexually mature female. However, Dunn (1998) found that males without calceoli tended to guard a suitable female for shorter periods of time. Although these results were preliminary, they nevertheless suggested that calceoli may play an indirect role in reproduction, possibly used to determine the moulting stage of females. The calceoli could, therefore, allow for the uptake of substances produced by the females just prior to moulting (Dunn 1998).
Besides the ambiguous results from behavioural experiments, the complex structure of calceoli and their orientation on the antennae has cast further doubt on their role as chemoreceptors. Other sensilla with a chemosensory function are described either as individual setae with little to no ornamentation or plumose setae with many setulae at the setae (Lincoln and Hurley 1981; Lincoln 1985; Mellon Jr 2014). Therefore, the alternative hypothesis of calceoli as a type of mechanoreceptor has been increasingly argued for (Lincoln and Hurley 1981; Lincoln 1985; Godfrey et al. 1988; Read and Williams 1990, 1991). Several studies focusing only on few species indicated that the calceoli are not directly innervated (Lincoln 1985; Godfrey et al. 1988; Read and Williams 1991).
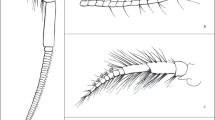
In this study, we integrated several methods including high magnification images from light, confocal laser scanning and scanning electron microscopes to gain a better understanding of the functional morphology of calceoli. For this approach, we acquired histological sections, confocal laser scanning as well as scanning electron micrographs of antennae with calceoli of the two species Gammarus locusta (Linnaeus 1758) (Fig. 1a) and Oediceroides calmani Walker 1906 (Fig. 1b). We discuss our findings in the light of the hypothesis that calceoli potentially function as mechanoreceptors.
Material and methods
Animals
Gammarus locusta (Linnaeus 1758) (Fig. 1a) and Oediceroides calmani Walker 1906 (Fig. 1b) were used for the experiments. These two species were selected because both have different lifestyles and differ significantly in the structure of their calceoli.
The lifecycle of G. locusta is strongly influenced by macroalgae like Ulva spp. and also Codium sp., Gigartina sp., Fucus sp. and Chaetomorpha sp., where the amphipods seek shelter and furthermore they use the algae as food source. Therefore, the populations are dependent on the algae density. These animals live between these macroalgae in shallow water up to 30 m depth. In the first months of the year in the northern hemisphere the G. locusta populations are scarce until April since the algae abundance is still scant. Then the density of the population increases in summer and in autumn the annual density peaks are reached until the density decreases again in winter just like the algae density. Depending on where the G. locusta population lives, it reproduces in warmer climates multiple generations, or in regions like the Baltic Sea only two generations per year, one in summer and one in winter (Costa and Costa 1999). In addition, these animals were available in sufficient quantity in the Zoological Collection of the LIB (Leibniz-Institut zur Analyse des Biodiversitätswandels – Standort Hamburg) (ZMH K-19800, collected from Kellenhusen in the Baltic Sea) as well as live material. The species G. locusta is native to Germany and was selected to also study innervation of the calceoli in freshly collected material. The live G. locusta were provided by Dr. Jan Beermann (Alfred-Wegener-Institute for Polar and Marine Research, Bremerhaven, Germany). Specimens were taken from the breeding tank and their sex was determined on site.
Seven specimens of O. calmani with well-developed calceoli were chosen from the specimens deposited in the collection of the LIB, collection number ZMH K-57386. The animals originated from the Southern Ocean (61°30.0′S 56°03.0′W) in up to 140 m depth and were collected during the Antarctic Expedition WH030C (WH75/3) in 1977–1978. Animals from this species can get up to 30 mm long and can be found in 15–550 m depth. In aquarium tests these animals buried themselves in sandy bottoms, so that the first antennules are erected, and the second antennae are skimming the surrounding sediment for food. The head and the upper part of the pereon reach out of the sand (Dauby et al. 2001). It was suggested that this species can alternate between predatory and detrivorous feeding mode, depending on the availability of food. In terms of diet and foraging behaviour, O. calmani is likely to be non-selective (Dauby et al. 2001).
Preparation
First, the second antennae had to be separated from the head for all microscopy methods that were used. The right second antenna was separated from each of the seven individuals of O. calmani which were used for section series, confocal laser scanning microscopy (CLSM), and scanning electron microscopy (SEM) analyses. Body parts (antennae or heads) were separated with a sharp scalpel under the stereomicroscope while the specimens were submerged in 80% ethanol.
A similar procedure was followed with the antennae of G. locusta. The live crustaceans were first relaxed with magnesium chloride and cooled at 4 °C before the antennae were separated and transferred to the fixative for the respective downstream analyses (see more details below).
Light microscopy
Habitus images and light microscopic details of calceoli of O. calmani and G. locusta were acquired with the Keyence VHX-5000 and VHX-7000 digital microscopes (Keyence, Japan). The animals were placed in an objective dish in 70% ethanol under the objective. For an optimal representation of the animals and the calceoli, the built-in focus stacking software was used.
Image of the slides of the histological sections were taken with the Axioskop2 together with the Axiocam 208 color (Zeiss, Germany).
Scanning electron microscope (SEM)
One head and at least one antenna from O. calmani was used for SEM analysis. The samples for SEM were transferred to a sample basket and dehydrated first in an ascending ethanol series and then 100% acetone before critical-point drying using a LEICA CPD 300. After drying, the samples were transferred to stubs with double-stick carbon stickers carefully under the stereomicroscope to tightly attach the specimens to the adhesive to reduce charging artefacts. The antennae were mounted so that the calceoli faced upward and could be imaged from different angles. Subsequently, the samples were coated with a thin platinum layer in a sputter coater (Polaron SC7650, Quorumtech, United Kingdom), and analysed and imaged using a LEO 1525 SEM (LEO Electron Microscopy Inc., United States) at the University of Hamburg, Germany. Magnification, accelerating voltage, and other settings were individually adjusted depending on the specimen.
Confocal laser scanning microscopy (CLSM)
Due to the size limitations, only the second antennae of both species were used for CLSM analyses. To increase cuticular fluorescence, all used specimens were stained using acid fuchsin. Acid fuchsin has been shown to stain the epicuticle of some Crustacean groups (Shelton and Chapman 1987), and it has been reported to improve imaging of cuticular details in amphipods (Timm et al. 2021). The powder was dissolved in 70% ethanol until the solution was saturated. The antennae were immersed in the dye and placed in the dark at room temperature for 24 h. Thereafter, the antennae were transferred first to an alcohol-glycerol mixture, followed by a series of changes with increasing glycerol content until the specimens were mounted in pure glycerol on microscope slides.
The samples were imaged using a Leica DM 2500 microscope with a Leica TCS SPE confocal setup (Leica, Germany) at the University of Hamburg with a laser emitting at a wavelength of 488 nm. PMT was set according to system’s setting, picking up roughly between 500 and 550 nm. Overview z-stacks of the whole antenna were taken using the 10 × objective, for z-stacks of the details of the calceoli the 40×- or even 63 × immersion objectives were used. “Detector gain” and “Amplitude offset” were set adjusted manually for each image to obtain maximal details. Acquired z-stacks were projected into 2D images for representations.
Histological sections
For the section series of O. calmani, antennae and one head with calceoli of two animals from the Hamburg Zoological Collection (catalogue numbers ZMH K 57386_1 and 57386_6) were used. For the section series of three basal antennal pieces bearing calceoli, three live G. locusta were fixed in Trumps reagent (2% formalin and 2% glutaraldehyde in 0.2 M cacodylate buffer, Electron Microscopy Science, catalogue number 11750). After fixation (and in the case of the museum material of O. calmani rehydration), specimens were post-fixed in 2% OsO4-solution in 0.2 M sodium cacodylate buffer, washed, dehydrated in an ascending ethanol series, transferred to acetone and embedded in Lowicryl. All steps were performed at room temperature. The gelatin capsules with the embedded specimens were polymerized for 48 h at 60 °C and then sectioned with 0.99 µm thickness using a Reichert Ultracut E ultramicrotome (now LEICA MICROSYSTEMS, Wetzlar, Germany). The series of sections were stained with 1% toluidine blue with 1% pyronine in 1% borax solution for 40 s at 60 °C. The final microscope slides were then sealed with Entellan.
Image processing
Clip Studio Paint Pro was used to create vector-drawings of calceoli and to edit the brightness and contrast of the images.
Results
Position and occurrence of calceoli in Gammarus locusta and Oediceroides calmani
The calceoli are located on the flagellum of the second antennae in both selected species. The calceoli are very small and translucent (Fig. 2), and often of similar size or smaller than the setae scattered throughout the antennae.
a Head (h), antenna 1 (a1) and antenna 2 (a2) of G. locusta ZMH K 19800. Scale bar: 500 µm; b Close-up of antenna 2 of G. locusta ZMH K 19800, the arrow is pointing to the fourth of five calceoli. Scale bar: 100 µm. c Head (h), antenna 1 (a1) and antenna 2 (a2) of O. calmani ZMH K-57386_2. Scale bar: 1 mm; d Close-up of antenna 2 focusing on calceoli (arrow) of O. calmani ZMH K-57386_2. Scale bar: 200 µm
Gammarus locusta
The calceoli are located on the dorsomedial side of the flagellum of the second antennae (Fig. 3a).
Each individual calceolus is located on one separate flagellar segment. The orientation of all calceoli is the same in that the proximal and distal elements point in the same direction, with the tip pointing away from the antenna.
Oediceroides calmani
The calceoli of O. calmani are located on the dorsomedial side of the flagellum (Fig. 4) of the second antennae. There is one calceolus on each segment of the flagellum. They are regularly arranged and similarly oriented (Fig. 4c); due to the length of the calceoli, they are arranged in a regular staggered order.
Calceolus-types
Since there already are detailed descriptions of the calceoli of G. locusta, we mainly highlighted new findings in this species and give a rough description for comparative purposes, while we here give a first detailed description of the calceoli in O. calmani.
Gammarus locusta
The calceolus of G. locusta belongs to the gammarid type and is 80–90 µm long and about 40–50 µm wide. It consists of two surface elements, which previous studies have referred to as the proximal and distal elements. The proximal element is crescent-shaped and slightly concave, whereas the distal element is defined by transverse overlapping cuticular ridges and has an oval shape (Fig. 5a). Furthermore, the calceolus consists of a basal receptacle that serves as a scaffold for the two surface elements and a stalk that connects the calceolus to the antenna (Fig. 5).
Overview of calceolus from G. locusta. a Maximum intensity projection of CLSM image stacks of a calceolus of G. locusta ZMH K 60041_a. Scale bar: 20 µm. b Longitudinal section through the base piece of the antenna and a calceolus from ZMH K 60041_c. Pictured is the proximal element (pe), a homogenous matrix (hm) inside of the receptacle (r) and the antenna (a) Scale bar: 20 µm. c Transverse section through the base piece of the antenna from ZMH K 60041_b. The calceolus with the proximal (pe) and distal elements (de), as well as the receptacle (r), bulla (b), and stalk (s) are visible. Within the receptacle there is also homogeneous matrix (hm). At the edge of the image, you can see the antenna (a). Scale bar: 20 µm
The sickle-shaped proximal element of G. locusta is adjacent to the proximal margin of the distal element, and not easily recognizable as an independent element. It has a weakly concave curvature, with the curved part looking as if it consists of closely spaced lamellae (Fig. 5a). It appears solid and closely associated with the receptacle in a broad area (Fig. 5). The internal structure of the distal element appears to be composed of fibre-like structure (Fig. 5b). The distal element consists of 20–25 transverse ridges and has an oval shape that narrows distally (Fig. 5a). In the sections, the element appears flat and is separated from the receptacle (Fig. 5c).
The receptacle connects all elements of the calceoli mentioned so far. In both SEM and CLSM images, the receptacle is barely visible due to the orientation of the calceoli, as it is largely obscured by the proximal and distal elements. Therefore, the histological sections provide the most detailed information about the structure. Based on the different staining intensity, it appears to be composed of different layers (Fig. 5c). Most of the layers have a structure similar to that of the cuticle of the antennae, suggesting that the stalk and underside of the receptacle are composed of cuticle with a similar organisation. However, the interior of the bulla appears to be composed of homogeneous matrix (Fig. 5b, c).
The stalk is broad and solid compared to the stalk of the calceoli of O. calmani and does not appear to have any neural innervation. The receptacle continues directly into the stalk (Fig. 5c), which is localized in a small cavity in the cuticle. Endo- and exocuticle can be differentiated by the different strength of the staining (Fig. 5b, c). Additionally, the stalk and cuticle in the cavity are darker in colour than the epicuticle at the other sites. The stalk of the calceolus can be seen to consist of endocuticle, exocuticle, and epicuticle, as does the cuticle of the antenna (Fig. 5c).
Oediceroides calmani
The calceoli of O. calmani belong to the oedicerotid type. The oval, bipartite distal and single cup-shaped proximal element is spatially separated, resulting in a different shape of the receptacle than in the gammarid calceolus type. It consists of two upwardly convex structures that are joined in the middle by the receptacle so that the overall structure appears tapered (Fig. 6). The calceolus itself is about 60 µm long and about 30 µm wide (Figs. 7, 8).
Schematic drawings of a calceolus of O. calmani ZMH K-57386_2. a Schematic drawing of a calceolus based on Fig. 8 a. The lamellae of the proximal element were not shown due to their high number. b Schematic reconstruction of the receptacle if the proximal and distal elements were removed
Overview of calceolus from O. calmani located on the second antennae. a Maximum intensity projection of a calceolus of O. calmani ZMH K-57386_8 (CLSM). The distal element (de), proximal element (pe) and stalk (s) can be seen. Scale bar: 20 µm. b Calceolus of O. calmani ZMH K-57386_2 (SEM). The distal element (de) as well as the proximal element (pe) visible. They are connected by the receptacle (r). Scale bar: 10 µm. c-d Longitudinal sections of the antenna of O. calmani ZMH K-57386_1. The inside of the distal element (de), proximal element (pe), receptacle (r), bulla (b) and stalk (s) are shown. The arrow points to the walls of the cavity that extends from the junction with the distal element to the basal side of the receptacle, where it is fused to it. Scale bar: 20 µm
Scanning electron micrographs of details from O. calmani ZMH K-57386 (SEM). a Lateral view of a calceolus with its receptacle of O. calmani ZMH K-57386_4. The arrow is pointing to the smooth surface of the bulla. Scale bar: 10 µm. b The distal element viewed from the side ZMH K-57386_4. Scale bar: 10 µm c View from above of a calceolus with a detached distal element ZMH K-57386_4. Scale bar: 10 µm. d The distal element viewed from above from ZMH K-57386_4. Scale bar: 10 µm. e A broken proximal element from ZMH K-57386_2. Scale bar:10 µm. f View of receptacle below proximal element of O. calmani ZMH K-57386_2. Close up on the cleft in the bulla. Next to the cleft is a wrinkled elevation on top of which the proximal cup sits. Scale bar:10 µm. g Broken distal element from ZMH K-57386_4. The arrow is pointing to the cuticular layers of the distal element. Scale bar: 10 µm. h Proximal element from ZMH K-57386_2. Scale bar: 10 µm. i Close up from the calceolus in C with its distal element missing ZMH K-57386_4. The arrow is pointing to the inside of the calceolus. Scale bar: 5 µm. j Stalk and its connection to the calceolus from ZMH K-57386_4. Scale bar: 5 µm
Particularly striking is the proximal element with its cup-shaped structure, which makes it remotely reminiscent of a satellite dish or a suction cup (Figs. 6, 7, 8). The round cup with a diameter of 20–25 µm sits freely in the receptacle and is only attached to it at its base (Figs. 6a, 8a, c, e, h). On the upper and lower surface of this cup lamellae are located which extend radially to the centre and terminate at the edge of the element (Fig. 8h). The proximal element is solid. In preparation artefacts the fractured area itself appears smooth in the SEM (Fig. 8e). The CLSM analyses reveal additional information on the structure of the proximal element: The outer edge of the cup appears toothed (Fig. 7a) and not as smooth as the one in the SEM image (Fig. 7b). The CLSM signal is weaker on the inner half of the cup than on the outer half of the cup (Fig. 7a).
The distal element is oval shaped and tapers distally (Fig. 8d). The elliptical surface is divided into two parts: The distal part consists of transverse ridges; in the proximal direction the proximal part fans out broadly and is covered with lamellae that strongly resemble the lamellar structure of the proximal element. Both areas are fused together and cannot be separated. Viewed from the side, the distal element is slightly concave and curved towards the antenna (Fig. 8b); its surface is characterized by radial ridges. Beneath the surface, cuticle layers are located that are oriented vertically to the element (Fig. 8g). This observation is confirmed by CLSM analysis, where the ribbed surface of the ridges is clearly visible (Fig. 7a). The ridges of the distal element stand out clearly due to the higher intensity, therefore, stronger fluorescence of the stain in the overlapping areas is visible. The same structure is present in the proximal region of the distal element since the lamellae emit a strong signal there. In some calceoli the crescent appears to be split in the middle of the proximal edge (Fig. 7a), but this might be due to the proximal edge of the distal element breaking under the pressure of the cover slip of the microscope slide.
The receptacle can be divided into two elements: a proximal area, which lies below the proximal element; a distal area that connects to the distal cup and the stalk. There is an opening leading into the structure in the distal area of the receptacle (Fig. 8i), and another opening is found proximal to it; they are separated by a hump-like bulge, the edge of which appears rolled up.
In sections, the receptacle appears cup-shaped surrounding the proximal element, with no direct contact, but it is connected to the stalk proximally (see below). Its surface is uneven and covered with spherical protrusions, and most characteristically it forms a bulge ventral and proximal to the proximal element, the so-called bulla. On the underside between the bulla and the beginning of the distal element, the surface of the receptacle is wrinkled and the spherical protrusions occur more frequently here (Fig. 8a). The bulla, on the other hand, has a mostly smooth surface (Fig. 8a). There is only a thin attachment from the distal element to the basal receptacle. Two stained lines can be seen in the distal region of the receptacle (Fig. 7c), probably forming the nodular bulge (Fig. 8i). These lines probably represent the walls of a cavity that extends from the junction with the distal element to the basal side of the receptacle, where it is fused to it (Fig. 6). The two lines probably form a cavity that begins below the distal element and eventually ends at the basal side of the receptacle. In most SEM images, a gap was apparent at the edge of the receptacle (Fig. 8), which suggested the existence of this cavity.
When viewed from above, the bulla can be observed as a cleft (Fig. 8f). The proximal element is positioned on a wrinkled elevation in the middle of the proximal receptacle area (Fig. 8f). The surface of the wrinkled elevation is more irregular than the surface of the surrounding receptacle. Dorsally, the surface of the receptacle appears to be a series of swirls: nodules, from which lamellae branch off in two directions at equal intervals, can be seen beneath the receptacle surface. The origin of the nodules is obscured by the proximal element. The structure ends in the area where the cup edge of the receptacle converges with the receptacle (Fig. 8f). The bulla is facing the antennal surface.
The stalk is not smooth and flat like that of the surrounding setae but appears branched and twisted. However, this could just be a drying artefact. It also appears to be composed of two parts: A solid part with an inconspicuous surface connected to the edge of the proximal end of the receptacle, and a part consisting of several interwoven structures. It is also noticeable that the stalk has small outgrowths almost like small hairs or scales. Although the presented data suggest that the calceolus is not hollow, no nerves or other signs of innervation could be identified.
Neural pathways in the antenna in close proximity to the calceolus
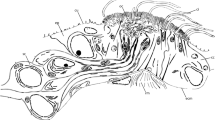
The inside of the antennae is mainly taken up by nervous tissue, muscles and supporting tissue. Longitudinal and transverse sections of the midpiece of the second antennae were analysed in this study, and both indicate nervous tissue extending through the segments of the antenna (Fig. 9). The cuticle below the base of the calceolus is thickened. This thick cuticle is located just below the stalk. The layer of less heavily coloured cuticle is endocuticle which extends into the stalk (Fig. 9).
a Histological cross-section of a base piece of the antenna in the area with the calceolus of G. locusta ZMH K 60041_b. The calceolus is laterally incised and the proximal (pe) as well as the distal element (de), the bulla (b), the stalk (s) and the receptacle (r) with homogeneous matrix (hm) can be seen. The openings of four setae in the cuticula are marked with arrows. There is also roundly shaped nervous tissue (n). Underneath the thickened cuticle of the calceolus is a darker coloured tissue that we suspect to be the putative calceoli nervous tissue (cn). Scale bar: 20 µm; b Histological longitudinal section of a base piece of the antenna of G. locusta ZMH K 60041_c. Only the receptacle (r) and the proximal element of the calceolus can be seen. On the inside of the antenna next to the cuticle is different looking putative calceoli nervous tissue (cn). Next to the calceoli is a seta (se) that is innervated by its putative dendrites (sn). Scale bar: 20 µm; c Higher magnification of the cross-section in a, taken with a different microscope, that focus on the cuticle thickening (ct) and the putative calceoli nervous tissue (cn). Scale bar: 20 µm; d Higher magnification of the longitudinal section that focuses on the darker tissue of the putative calceolus nervous tissue (cn) and the innervation of the setae (sn). Scale bar: 20 µm
There is a darker coloured tissue below the calceolus that is reminiscent of a neurite bundle. This potential nervous tissue covers the area directly beneath the thickened cuticle underneath the calceolus and reaches into the direction of the center of the antenna (Fig. 9a).
Four setae are also visible in the transverse section of the antenna, with structures morphologically strongly resembling dendrites that are most likely passing through the holes in the cuticle (Fig. 9a). The possible dendrites of the setae are coloured lightly (Fig. 9d marked by arrows).
Discussion
This study provides the first detailed information on the calceoli of O. calmani and furthermore is the first to investigate innervation not only in the structure of the calceolus, but also directly underneath it within the second antenna of amphipods.
Morphological differences of the calceolus types in the two investigated species
The calceoli of the species G. locusta and O. calmani belong to different calceoli types, and their morphology differs significantly (Fig. 10). According to the study by Lincoln and Hurley (1981), which was based mainly on SEM-data, the calceoli of G. locusta belong to the gammarid type, which is supposedly the simplest type of calceoli, while O. calmani possesses the oedicerotid type.
Schematic drawings of the two calceoli types used in this study. The lamellae of the proximal element in both calceoli types were not shown due to their high number. a Schematic drawing of the gammarid type from G. locusta. Based on ZMH K 60041_a; b Schematic drawing of a calceolus with the oedicerotid-type based on O. calmani ZMH K-57386_2
The most striking difference between the two calceoli types is the structure of the proximal element: It is cup-shaped and sits separately on the receptacle in O. calmani (Fig. 7), whereas it is sickle-shaped and sits directly against the distal element in G. locusta (Fig. 5). Based on our analyses, the distal element of O. calmani is very complex and resembles a fused variant of the distal and proximal elements of G. locusta (Fig. 5).
The orientation of the calceoli along the antenna and the stalk of the two types also differ. Calceoli of G. locusta have a solid stalk, they are less numerous than in O. calmani and are oriented perpendicular to the antennal surface (Fig. 3). In contrast, the calceoli of O. calmani are always oriented towards the tip of the antenna and have a thinner stalk than in G. locusta (Fig. 4). The receptacle of O. calmani has a more complex structure than that of G. locusta and can have prominent and structurally different distal and proximal parts. Both areas differ from each other in their structure (Fig. 10). These observations suggest that the more complex type of Oedicerotidae evolved by duplication or separation of the proximal and distal elements of a simpler calceolus type.
Another difference between the two calceoli types is that there are several cavities in the receptacle of O. calmani which are absent from the gammarid type: A prominent feature is the deepening of the bulla, which is located close to the proximal element. Below the distal element is an opening (Fig. 8) which predictably continues into the interior of the receptacle (Fig. 7c). Such cavities have not been found in G. locusta, further indicating the higher complexity of the oedicerotid type of calceolus.
A potential explanation for the different types of calceoli in these two species, or in amphipods in general could be found in the habitat. In low-light environments, such as the deep sea, hydrodynamic receptors are very important as they can help locate predators and potential mates in the dark. As O. calmani lives in the deep sea, it is possible that low light conditions have influenced the evolution of a complex calceoli type. Oediceroides calmani has significantly more calceoli than G. locusta with the simpler calceolus type. In its detrivorous lifestyle O. calmani is partly buried in the sand, it uses their second antenna to skim the sediment for food (Dauby et al. 2001). The antennae probably have to collect a lot of information of the surrounding sediment to the amphipod for its survival. Perhaps the more complex form of the calceolus can provide information about the surrounding sediment. Also, by being partially buried, the animal needs to gather more information on the water movement in its environment, so that it can detect approaching predators or conspecifics earlier.
What kind of sensory organ could calceoli be?
Our initial aim was to further test the hypothesis that calceoli have a mechanosensory function as proposed by Read and Williams (1990) by investigating possible direct or indirect neural innervation of this structure. Our histological sections through the antennae of freshly fixed material of G. locusta indicate that there is no nervous tissue inside of the stalk or in any other part of the calceoli; the calceoli are, therefore, identified as an entirely cuticular formation. It cannot be excluded that there is not a single axon that is associated with the inside of the stalk or the calceolus based on the techniques and their limitations used in this study. There is darker coloured tissue (labelled cn in Fig. 9a, c) below the calceolus that is reminiscent of a bundle of nervous tissue (Fig. 9). We suspect that these might be receptor cells because they are the first neurons in a signal transduction chain of sensory stimuli (Richter et al. 2010). This potential nervous tissue is next to the cuticle thickening at the base of the calceolus and extends from there into the interior of the antenna, although we could not trace it much further.
Several different sensilla have been described in crustacean species, but none resembles the complex structure of calceoli. The described sensory cells give crustaceans the ability to see, smell, recognise a potential mate, detect a predator or prey, all of which are essential for survival. Among the most common sensilla we distinguish between a chemosensory function and a mechanoreceptory function (Mellon 2014).
There are different types of mechanoreceptors. Mechanoreceptive setae for example are located on nearly the whole body of malacostracans, from the antennae to the uropods. The exclusively mechanoreceptive sensilla are divided into two main categories: The hydrodynamic (near-field) receptors and contact mechanoreceptors. Hydrodynamic receptors appear nearly on the whole body from the antennules over the cephalothorax to the uropods, e.g. hair-peg and hair-fan receptors are organs sensitive to water movement (Mellon 2014). These mechanoreceptors in water are sensitive to water deformation and act as high-pass filters to determine sensitivity to hydrodynamic stimuli. Mechanosensory signals are relevant for feeding, social behaviour and predator–prey interactions, especially if the surrounding light or visibility is poor (Lenz and Hartline 2014; Mellon 2014). In general, a mechanoreceptor must be physically moved, and this movement stretches a receptor dendrite containing stretch–sensitive ion channels. A common form of mechanoreceptors found in crustaceans are setae and feathered sensilla. A characteristic attachment point of the dendrite is located in the basal end of the setae. Thus, the dendrite is stretched as soon as the setae is bent (Lenz and Hartline 2014).
If calceoli were mechanoreceptors, they would have to be movable, and the associated dendrites would have to be stretched to transmit a signal. However, unlike mechanosensory sensilla, calceoli most likely do not have nervous tissue within the stalk or directly at its base as indicated by this and earlier studies; furthermore, our study reveals a continuous cuticle between antenna and calceolus-stalk, thereby making an innervation site for nerves as described for chemosensory or mechanosensory setae unlikely. Thus, it would probably not be sufficient to bend a calceolus to transmit a signal, as is the case with bimodal sensilla for example. However, we show nervous material accumulated underneath the cuticle of the stalk of the calceolus (Fig. 9). Tolouidine blue-stained cuticle below the stalk, potentially forms a structure where the nervous tissue is connected to the stalk (Fig. 9a). With experimental confirmation still pending, it could be possible that hydrodynamic stimuli in the water cause the calceolus to vibrate and that this movement would be transmitted through the stalk to the hemispherical cuticular formation in the antenna below the stalk (Godfrey et al. 1988). Those movements would in turn stretch the attached neurons resulting in them translating the movement into an action potential, that could be transmitted to the central nervous system.
Alternatively, calceoli were also suggested to be chemosensory organs before (Dahl et al. 1970; Dunn 1998). The calceoli up until now have only been found on the antennae, so the calceoli are very likely to have a function that helps to identify information sampled from the environment around them. Because of their position and expression in adult males it is reasonable to assume that the calceoli might have a chemosensory function. Crustaceans pick up chemical signals from the environment mainly with their aesthetascs, which are located only on the antenna, or with sensilla, which are scattered across the whole body (Thiel and Breithaupt 2011). The antennae are thereby able to actively sample the environment and detect potential dangers, conspecifics, sexual partners, or prey in time based on the sensory impressions thus obtained (Staudacher et al. 2005). Chemosensory sensilla are divided into two main chemosensory systems: Distributed chemoreception where the chemosensitive sensilla respond to chemosensory and mechanical stimuli; and the olfactory sense that is used to detect odorants in the surrounding medium. (Mellon 2014; Sandeman et al. 2014) The before mentioned aesthetascs belong to the olfactory sense and are associated with the flagellum of the first antenna. They are 100–600 µm in length depending on the species (Mellon 2014). In the past, it was suspected that calceoli might be a variant of the aesthetascs. It was not until species were observed in which calceoli are also present on the first antenna that it became clear that they were not modified aesthetascs (Lincoln and Hurley 1981), since these normally have a very simple structure and a spongy cuticle to keep the neurons more directly connected to the environment (Hallberg and Skog 2011). The calceoli investigated in this study appear as if they possess a solid surface that is not composed of permeable cuticle. Histological sections have shown that calceoli, and their stalks of G. locusta are composed of cuticle. In addition, there is a thickening of the cuticle directly at the base of the calceolus. The nervous tissue attaches below the base. This would make it difficult for the calceoli to absorb chemicals from the environment, which is why we consider a purely chemosensory function of the calceoli to be unlikely.
Bimodal chemo-mechanosensory are sensilla like the hooded sensilla (Cate and Derby 2002), hedgehog sensilla (Derby 1982) and the asymmetric hair (Schmidt and Derby 2005). They combine a chemosensory and a mechanosensory pathway. Bimodal sensilla can also occur on both flagella of the first antennules (Mellon 2007). The hair-peg organs, that also function as a hydrodynamic receptor, belong also to the bimodal sensilla (Schmidt 1989). This shows that chemosensory and mechanosensory function within a sensilla need not be mutually exclusive. Although we found no evidence for a chemosensory pathway in our analyses of the calceoli, this does not mean that the possibility of the calceoli being bimodal sensilla can be completely ruled out.
How could calceoli function?
An interesting feature of the calceoli is their orientation along the antennae. All Calceoli sit at the same distance from one another on the antennae and face in the same direction. They are regularly distributed on the antennae. In G. locusta and O. calmani the calceoli point anteriorly and are located on the dorsal side of the antenna. The first antennule and the second antennae in pelagic crustaceans play an important role when it comes to detecting chemical cues and distance perception (Lenz and Hartline 2014). The long antennae are facing anteriorly so that the distance of the sensilla associated with the antennae to the animal body is increased. With this distance the localization of stimuli can be improved, since they are isolated from the water movements created by the other swimming appendages (Lenz and Hartline 2014). Also, there were no muscle fibres found in the calceoli, suggesting that the animals have no active influence on the orientation of the calceoli on the antenna. Therefore, the orientation of the calceoli to the antennae is fixed. All calceoli point with the lamellate side in the same direction (Figs. 3, 4). This feature is interesting because it is an indication that the orientation may have a meaning that we do not yet know. Such directionality is also an important property of vibrio receptors (Read and Williams 1991), which must be able to locate, e.g., hydrodynamic stimuli from the environment.
It could be expected that the calceoli have a chemoreceptor function since they appear in most species exclusively in males, as there is often sexual dimorphism in chemosensory systems (Derby and Weissburg 2014). When it comes to sexual dimorphism the adult males, but not in the juvenile stages, have more chemoreceptors than the females (Derby and Weissburg 2014). The calceoli are also more abundant on adult males than on juveniles. Chemosensory signaling in mating is known for most crustacean groups. Some studies that have investigated this issue have removed a large part of the antenna to investigate the relevance of this sexual dimorphic structure in mating like Read and Williams (1990) have done for investigating the calceoli. The most common difference in sexual dimorphism is the number of chemosensors. Many species of Amphipoda males have a greater number of hair-like setae on the antennules than females (Bauer 2011). The same is true for the calceoli (Hurley 1980). We found no evidence for a chemosensory function, but a function important for mating behaviour is a likely explanation for calceoli.
The formation of calceoli is probably related to the sexual maturity of males in some species. Read and Williams (1991) observed in Gammarus pseudolimnaeus (Bousfield 1958), that the size of the ridges of the distal element seems to be the same in small and big sexually mature males and is related to their sexual maturity. They further found evidence that calceoli are only fully developed in males after they reached sexual maturity, so likely the number and size of the ridges as well as the space between them is important for their function. Perhaps it allows them to resonate at a biologically important frequency (Read and Williams 1991).
We, therefore, agree with Read and Williams (1991) on their suggestion that calceoli could potentially resonate only at certain frequencies, thereby evaluating a signal from the environment that is not significant until adulthood. Although there are different types of calceoli throughout the families, their function could be the same. It cannot be excluded that the calceoli could be a bimodal sensilla, since bimodal sensilla are known to be morphological complex like the hedgehog sensilla (Derby 1982) and the hooded sensilla (Cate and Derby 2002).
Conclusion
We consider an exclusively chemosensory function of calceoli to be unlikely. We assume that the calceoli are functioning either as hydrodynamic receptors and perceive hydrodynamic stimuli or as bimodal sensilla and perceive chemosensory and mechanosensory signals.
Data availability
All relevant data is presented in the manuscript. Raw image data can be made available upon request to the authors.
References
Bellan-Santini D (2015) Order Amphipoda Latreille, 1816. In: von Vaupel Klein JC, Charmantier M, Schram FR (eds) Treatise on zoology-anatomy, taxonomy, biology. The Crustacea, vol 5. Brill, Leiden, pp 93–248
Cate HS, Derby CD (2002) Hooded sensilla homologues: structural variations of a widely distributed bimodal chemomechanosensillum. J Comp Neurol 444:345–357. https://doi.org/10.1002/cne.10153
Cole GA (1970) Gammarus minus: geographic variation and description of new subspecies G. m. pinicollis (Crustacea, Amphipoda). Trans Am Microsc Soc. https://doi.org/10.2307/3224561
Costa FO, Costa MH (1999) Life history of the amphipod Gammarus locusta in the Sado estuary (Portugal). Acta Oecol 20:305–314. https://doi.org/10.1016/S1146-609X(99)00136-8
Croker RA, Gable MF (1977) Geographic variation in Western Atlantic populations of Gammarus Oceanicus Segerstrale (Amphipoda) 1. Crustaceana 32:55–76
Dahl E, Emanuelsson H, von Mecklenburg C (1970) Pheromone transport and reception in an amphipod. Science 170:739–740. https://doi.org/10.1126/science.170.3959.739
Dauby P, Scailteur Y, De Broyer C (2001) Trophic diversity within the eastern Weddell Sea amphipod community. Hydrobiologia 443:69–86. https://doi.org/10.1023/A:1017596120422
Derby CD (1982) Structure and function of cuticular sensilla of the lobster Homarus americanus. J Crustac Biol 2:1–21. https://doi.org/10.2307/1548106
Derby CD, Weissburg MJ (2014) The chemical senses and chemosensory ecology of crustaceans. In: Derby C, Thiel M (eds) The natural history of the Crustacea: nervous systems and control of behavior, vol 3. Oxford University Press, pp 263–293
Dunn AM (1998) The role of calceoli in mate assessment and precopula guarding in Gammarus. Anim Behav 56:1471–1475. https://doi.org/10.1006/anbe.1998.0916
Godfrey RB, Holsinger JR, Carson KA (1988) A comparison of the morphology of calceoli in the freshwater amphipods Crangonyx richmondensis s. lat. (Crangonyctidae) and Gammarus minus (Gammaridae). Crustaceana. Supplement 115–121
Hallberg E, Skog M (2011) Chemosensory Sensilla in Crustaceans. In: Thiel M, Breithaupt T (eds) Chemical communication in crustaceans. Springer, Heidelberg, pp 103–122. https://doi.org/10.1007/978-0-387-77101-4_6
Hartnoll RG, Smith SM (1980) An experimental study of sex discrimination and pair formation in Gammarus duebenii (Amphipoda). Crustaceana 253–264
Hurley DE (1980) A provisional checklist of Crustacea Amphipoda known to have calceoli. N Z Oceanogr Inst 4:71–120
Lenz PH, Hartline DK (2014) Mechanoreception in crustaceans of the pelagic realm. In: Derby C, Thiel M (eds) The natural history of the Crustacea: nervous systems and control of behavior, vol 3. Oxford University Press, pp 293–320
Lincoln RJ (1985) Morphology of a calceolus, an antennal receptor of gammaridean Amphipoda (Crustacea). J Nat Hist 19:921–927. https://doi.org/10.1080/00222938500770571
Lincoln RJ, Hurley DE (1981) The calceolus, a sensory structure of gammaridean amphipods (Amphipoda: Gammaridea). Bull Br Mus Nat Hist Zool 40:103–116
Linnaeus C (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata [10th revised edition], vol 1, pp 824
Mekhanikova IV (2021) Calceoli: antennal sensory organs of Amphipods (Crustacea, Amphipoda, Gammaridea) from Lake Baikal. Biol Bull 48:1250–1262. https://doi.org/10.1134/S1062359021080215
Mellon D Jr (2007) Combining dissimilar senses: central processing of hydrodynamic and chemosensory inputs in aquatic crustaceans. Biol Bull 213:1–11. https://doi.org/10.2307/25066612
Mellon D Jr (2014) Sensory systems of crustaceans. In: Derby C, Thiel M (eds) The natural history of the Crustacea: nervous systems and control of behavior, vol 3. Oxford University Press, pp 49–84
Milne-Edwards H (1830) Extrait de recherches pour servir à l’histoire naturelle des crustacés amphipodes [Research excerpt for use in the natural history of amphipods (Crustacea)]. Dict Class D’hist Nat 16:511
Read AT, Williams DD (1990) The role of the calceoli in precopulatory behaviour and mate recognition of Gammarus pseudolimnaeus Bousfield (Crustacea, Amphipoda). J Nat Hist 24:351–359. https://doi.org/10.1080/00222939000770261
Read AT, Williams DD (1991) The distribution, external morphology, and presumptive function of the surface microstructures of Gammarus pseudolimnaeus (Crustacea: Amphipoda), with emphasis on the calceolus. Can J Zool 69:853–865. https://doi.org/10.1139/z91-129
Richter S, Loesel R, Purschke G, Schmidt-Rhaesa A, Scholtz G, Stach T, Vogt L, Wanninger A, Brenneis G, Döring C, Faller S (2010) Invertebrate neurophylogeny: suggested terms and definitions for a neuroanatomical glossary. Front Zool 7:1–49. https://doi.org/10.1186/1742-9994-7-29
Sandeman DC, Kenning M, Harzsch S (2014) Adaptive trends in malacostracan brain form and function related to behavior. In: Derby C, Thiel M (eds) The natural history of the Crustacea: nervous systems and control of behavior, vol 3. Oxford University Press, pp 11–48
Schmidt M (1989) The hair-peg organs of the shore crab, Carcinus maenas (Crustacea, Decapoda): ultrastructure and functional properties of sensilla sensitive to changes in seawater concentration. Cell Tissue Res 257:609–621. https://doi.org/10.1007/BF00221472
Schmidt M, Derby CD (2005) Non-olfactory chemoreceptors in asymmetric setae activate antennular grooming behavior in the Caribbean spiny lobster Panulirus argus. J Exp Biol 208:233–248. https://doi.org/10.1242/jeb.01357
Shelton PMJ, Chapman CJ (1987) A living tag for recording moult histories in crustaceans. ICES J Mar Sci 43:209–215. https://doi.org/10.1093/icesjms/43.3.209
Staudacher EM, Gebhardt M, Dürr V (2005) Antennal movements and mechanoreception: neurobiology of active tactile sensors. Adv Insect Physiol 32:49–205. https://doi.org/10.1016/S0065-2806(05)32002-9
Thiel M, Breithaupt T (2011) Chemical Communication in Crustaceans: research challenges for the twenty-first century. In: Thiel M, Breithaupt T (eds) Chemical communication in crustaceans. Springer, Heidelberg, pp 3–22. https://doi.org/10.1007/978-0-387-77101-4_1
Timm R, Schwentner M, Bober S, Lörz AN (2021) Testing the impact of non-destructive DNA extraction on setae structure of Amphipoda (Crustacea). Zootaxa. https://doi.org/10.11646/zootaxa.4950.1.10
Tomikawa K, Kimura N (2021) On the brink of extinction: a new freshwater amphipod Jesogammarusa calceolus (Anisogammaridae) from Japan. ZooKeys 1065:81–100. https://doi.org/10.3897/zookeys.1065.71687
Walker AO (1906) Preliminary descriptions of new species of Amphipoda from the “Discovery” Antarctic Expedition, 1902–1904. Ann Mag Nat Hist (Ser 7) 18:13–18
Acknowledgements
The authors would like to thank the Museum der Natur Hamburg of the Leibniz-Institut zur Analyse des Biodiversitätswandels in Hamburg for allowing us to access the material. We are thankful for Jan Beermann (Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung) who kindly provided fresh samples. We gratefully thank Renate Walter, Sabine Gaude, Frank Friedrich, Simon Bober, Stephanie Köhnk, and Frederik Jessen (University of Hamburg, Leibniz Institut, Hamburg), for the assistance in the morphological laboratory. Anne-Nina Lörz is funded by the German Research Foundation project IceAGE Amphipoda (LO2543/1-1). Open Access was supported by the University Hamburg DEAL.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.T. conducted the lab work and prepared the figures. All authors, R.T., A.K. and AN.L. discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interes
A.K. is a member of the editorial board of the journal Zoomorphology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Timm, R., Kerbl, A. & Lörz, AN. New insights into the functional morphology of calceoli in Amphipoda (Crustacea). Zoomorphology (2024). https://doi.org/10.1007/s00435-024-00645-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00435-024-00645-8