Abstract
Objective
The aim of this study is to conduct a systematic evaluation of the diagnostic efficacy of Breast Imaging Reporting and Data System (BI-RADS) 4 benign and malignant breast lesions using magnetic resonance imaging (MRI) radiomics.
Methods
A systematic search identified relevant studies. Eligible studies were screened, assessed for quality, and analyzed for diagnostic accuracy. Subgroup and sensitivity analyses explored heterogeneity, while publication bias, clinical relevance and threshold effect were evaluated.
Results
This study analyzed a total of 11 studies involving 1,915 lesions in 1,893 patients with BI-RADS 4 classification. The results showed that the combined sensitivity and specificity of MRI radiomics for diagnosing BI-RADS 4 lesions were 0.88 (95% CI 0.83–0.92) and 0.79 (95% CI 0.72–0.84). The positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were 4.2 (95% CI 3.1–5.7), 0.15 (95% CI: 0.10–0.22), and 29.0 (95% CI 15–55). The summary receiver operating characteristic (SROC) analysis yielded an area under the curve (AUC) of 0.90 (95% CI 0.87–0.92), indicating good diagnostic performance. The study found no significant threshold effect or publication bias, and heterogeneity among studies was attributed to various factors like feature selection algorithm, radiomics algorithms, etc. Overall, the results suggest that MRI radiomics has the potential to improve the diagnostic accuracy of BI-RADS 4 lesions and enhance patient outcomes.
Conclusion
MRI-based radiomics is highly effective in diagnosing BI-RADS 4 benign and malignant breast lesions, enabling improving patients’ medical outcomes and quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) remains a prevalent malignancy worldwide, accounting for approximately 31% of all cancer cases in women, with a mortality rate showing an annual increase of 415 (Siegel et al. 2023). In line with the breast cancer screening guidelines provided by the National Comprehensive Cancer Network (NCCN) and the Breast Imaging Reporting and Data System (BI-RADS), suspicious breast lesions are typically classified into six distinct categories (Goetz et al. 2019). Among these categories, BI-RADS 4 breast lesions are characterized as being suspicious for malignancy with an unknown pathological type, presenting a wide range of malignancy probabilities ranging from 2 to 95% (including 4a:2–10%,4b:10–50%,4c:50–95%). To further investigate and establish an accurate diagnosis, needle biopsy is commonly performed as a diagnostic procedure (Bennani-Baiti et al. 2017). However, the absence of clear qualitative characteristics for BI-RADS 4 breast lesions has led to overdiagnosis and unnecessary needle biopsies, causing physical harm to patients. Like any clinical diagnostic method, there is a chance of false positives and missed diagnoses (Gradishar et al. 2017). Therefore, the development of noninvasive and efficient diagnostic methods for breast BI-RADS 4 lesions holds great significance.
Breast magnetic resonance imaging (MRI) has emerged as a crucial tool for evaluating and detecting breast lesions. In routine clinical examinations, it is widely employed alongside mammography and ultrasonography to diagnose breast cancer. It provides vital information regarding tumor size, location, and invasion extent, aiding in the assessment of lymph node and tissue involvement. (Sohn and Bisdas 2020). Radiomics is an emerging approach that involves extracting high-throughput image features from medical images and conducting quantitative analysis. This is done by annotating abnormal findings and subsequently applying routine image processing techniques, which helps in obtaining diagnostic outcomes. Due to its capacity to enhance the diagnostic accuracy of radiologists, radiomics has garnered significant attention in the field of diagnostic image analysis (Ye et al. 2020). While there is significant research backing the use of MRI radiomics in diagnosing benign and malignant breast lesions (Satake et al. 2022), previous studies examining the effectiveness of MRI radiomics in diagnosing BI-RADS 4 lesions have been hindered by small sample sizes, limited statistical analyses, and inconsistent research findings. Therefore, a comprehensive meta-analysis is warranted to assess the diagnostic efficacy of MRI radiomics in differentiating between benign and malignant breast lesions categorized as BI-RADS 4.
Materials and methods
The meta-analysis was conducted following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (McInnes et al. 2019).
Literature search
In this study, LJ and ZH conducted a systematic search across four publicly available databases: PubMed, Embase, Cochrane Library, and Sinomed. The search included studies published until May 17, 2023, employing a Boolean algorithm with the following keywords: ((machine learning OR texture OR artificial intelligence OR radiomics) AND (((magnetic resonance OR MRI OR MRI Scan OR Magnetic Resonance Imaging OR Magnetic Resonance Tomography)) AND ((Breast Lesion OR Breast Tumor OR suspicious breast lesion OR breast mass)) AND ((Breast Imaging Reporting and Data System OR Breast Imaging Reporting and Data System score OR BI-RADS)). The reviewers then independently evaluated the titles and abstracts of the identified articles to determine their eligibility. To ensure the quality of the research, we excluded case reports, nonoriginal investigations (such as editorials, letters, and reviews), and studies that were not relevant to the research topic. In cases where there were differences in opinion between the two reviewers, we they resolved them through constructive discussion and came to a consensus.
Literature inclusion and exclusion criteria
Two reviewers, adhering to the PICO (Population, Intervention, Comparison, Outcome) principles, developed specific inclusion and exclusion criteria to identify eligible studies for inclusion in the meta-analysis. Inclusion criteria are as follow: (1) The literature included subjects with breast lesions diagnosed as either benign or malignant based on pathological criteria. (2) The subjects underwent breast MRI examination prior to undergoing biopsy or excision surgery. (3) All subjects in the literature were diagnosed with BI-RADS 4 breast lesions following MRI examination. (4) The studies involved radiomics analysis conducted on breast MRI images. Exclusion Criteria are as follow: (1) The subjects included in the study exhibited breast lesions that were not categorized as BI-RADS 4 following MRI assessment. (2) The subjects underwent biopsy or surgical resection prior to the MRI examination. (3) The availability of relevant data was limited or incomplete despite attempts to contact the authors.
Literature screening and data extraction
Two reviewers used EndNote (v20.0) to import and removed duplicate records from the retrieved literature. The reviewers then conducted an initial screening of titles and abstracts to identify original research data that met the criteria. The selected data was then downloaded for further in-depth reading and analysis. When the included literature had limited or incomplete data, two reviewers contacted the original authors via email to request complete data. If obtaining complete data was not possible, the paper was excluded from the analysis. The final set of literature included in this meta-analysis was determined based on the predefined inclusion and exclusion criteria.
Prior to data extraction, two reviewers developed a standardized extraction form that included various parameters such as article title, author names, publication year, author’s country, study design, MRI equipment manufacturer, MRI magnetic field strength, feature screening algorithm, radiomics algorithm, contrast agent type, and ratio of the test set to the training set. The reviewers extracted the values of true positive (TP), false positive (FP), true negative (TN), and false negative (FN) from the data to generate a 2 × 2 contingency table. In cases where multiple radiomics models were reported in a single study, the model with the highest diagnostic accuracy was selected for meta-analysis. Any disagreements between the two reviewers were resolved through discussion until a consensus was reached.
Data quality assessment
The selected studies will undergo quality assessment using the Radiomics Quality Score (RQS) scale, originally proposed by Lambin et al. in 2017. This scale comprises 16 items that evaluate various aspects, including image acquisition, radiomics feature extraction, data modeling, model validation, and data sharing. Each item is assigned a specific score based on the extent to which the research fulfills the criteria. The score range is from – 8 to 36 (Lambin et al. 2017), which is subsequently converted into a percentage score ranging from 0 to 100%. Scores from – 8 to 0 are considered as 0%, while a score of 36 is considered as 100%. To ensure a comprehensive assessment of study quality in this meta-analysis, the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) scale is also employed to evaluate the risk of bias in the included studies (Whiting et al. 2011).
Statistical analysis
In this study, Meta-Disc 1.4 software was utilized to conduct a heterogeneity test, and the threshold effect was assessed by calculating Spearman’s correlation coefficient. A threshold effect was considered present when the calculated P value was less than 0.05 (Devillé et al. 2002). The heterogeneity among the included studies was evaluated using the Cochran Q test and the I2 statistic. Statistical significance was determined when the P value was less than 0.05, and a value of I2 greater than or equal to 50% indicated moderate to high heterogeneity across the studies (DerSimonian and Laird 1986).
This study calculated sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with 95% confidence intervals (95% CI). Summary receiver operating characteristic (SROC) analysis generated the area under the curve (AUC). A sensitivity analysis excluded two studies that only analyzed the test set. Publication bias was assessed using a Deeks funnel plot, with a slope coefficient’s P value below 0.10 indicating significant publication bias (Deeks et al. 2005). Statistical analyses utilized STATA 16.0’s ‘midas’ module.
Results
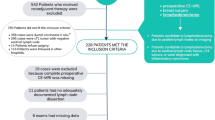
A comprehensive search across four public databases initially identified 2,012 records. After removing 943 duplicate documents, a meticulous evaluation of titles, abstracts, and full-text articles led to the exclusion of 1,049 studies, encompassing reviews, letters, conference reports, and other publications unrelated to the subject matter. Subsequent re-screening of the remaining 20 literature sources was performed based on predefined inclusion and exclusion criteria. Consequently, 9 articles were found ineligible and excluded from the analysis. Finally, 11 articles meeting the defined criteria were included in the meta-analysis (Zhang et al. 2022). The PRISMA flowchart below visually illustrates the study selection process (Fig. 1).
Study overview
This meta-analysis analyzed 11 studies (Ellmann et al. 2020; Hu et al. 2017; Bickelhaupt et al. 2018; Yin et al. 2022; Hu et al. 2023; Zhao et al. 2021; Zhou et al. 2021; Bakker et al. 2019; Liu et al. 2019; Rao et al. 2016; Thibault et al. 2013a) published between December 2017 and March 2023, which collectively involved 2208 patients with 2230 lesions identified as BI-RADS 4. The omics method was used to evaluate 1915 lesions in 1893 patients, reflecting the practical application of imaging techniques. Among the included studies, 8 were from China, while the remaining 3 were conducted in Austria, Germany, and the United States. Machine learning algorithms were utilized in 5 studies, deep learning algorithms in 3 studies, and the algorithms used in the remaining 3 studies were not explicitly specified. The table below presents a detailed summary of the radiomics and associated imaging data extracted from the 11 studies included in the analysis (Table 1).
RQS and QUADAS-2 assessment
The included studies exhibited a median RQS score of 20, ranging from 13 to 23 points, representing 55% of the total RQS score, which ranged from 36 to 63%. Notably, one study attained the highest score of 23 points (63%) (Bickelhaupt et al. 2018), while two studies received the lowest score of 13 points (36%) (Bickelhaupt et al. 2018; Yin et al. 2022) due to their failure to conduct internal and external validation. In terms of individual item assessment, all studies met the criteria for eight indicators: image protocol, multiple segmentation, feature reduction, cutoff determination, calibration, discrimination and resampling, gold standard reference, and clinical utility. However, the indicators related to the phantom study, prospective study, cost-effectiveness, biological correlates, nonradiomics factors, and multiple time points received a score of 0, as none of the included studies addressed these specific aspects. To ensure a comprehensive assessment of study quality in this meta-analysis, the QUADAS-2 scale is also employed to evaluate the risk of bias in the included studies (Whiting et al. 2011). According to the QUADAS-2 assessment, most of the studies had low risk of bias and limited applicability concerns, indicating high-quality data. A comprehensive summary of the RQS scores and a detailed summary of the risk of bias and applicability concerns for all the studies included can be found in Fig. 2.
MRI radiomics-based diagnosis of BI-RADS 4 benign and malignant breast lesions
The threshold analysis revealed no threshold effect between MRI radiomics-based diagnosis of benign and malignant breast lesions in BI-RADS 4, with a correlation coefficient of r = – 0.246 (p = 0.466). The study included 1893 patients with a total of 1915 lesions, out of which 1351 were malignant and 570 were benign. The combined findings are presented in Fig. 3, illustrating a forest plot. The combined results are visually presented in Fig. 4, which displays a SROC diagram. The combined sensitivity was 0.88 (95% CI 0.83–0.92, I2 = 76.44%), specificity was 0.79 (95% CI 0.72–0.84, I2 = 55.72%), PLR was 4.2 (95% CI 3.1–5.7), NLR was 0.15 (95% CI 0.10–0.22), DOR was 29.0 (95%CI 15–55), and AUC based on SROC curve was 0.90 (95% CI 0.87–0.92), indicati1ng a high diagnostic value.
Subgroup and sensitivity analyses
Subgroup analyses were conducted on sixteen subgroups across eight distinct conditions. These conditions included race (Asian and non-Asian), training set population to test set population ratio (7/3 and non-7/3), feature selection algorithm (LASSO and non-LASSO), contrast agent (gadobenate dimeglumine and non-gadobenate dimeglumine), tumor segmentation (manual and automatic), MRI equipment (Siemens and GE), magnetic field strength (3.0 T and 1.5 T), and radiomics algorithms (machine learning and deep learning).
Regression analysis revealed significant sources of heterogeneity in all conditions, with the exception of race. Detailed illustrations of these findings are presented in the accompanying figure and table below (Fig. 5; Table 2).
In this study, a sensitivity analysis was conducted by excluding studies that only performed radiomics analysis on the test set data (Zhao et al. 2021; Bakker et al. 2019). The data was then re-analyzed, and it was found that there were no significant changes in sensitivity (0.88 vs 0.88) and specificity (0.79 vs 0.77). In addition, an exclusion analysis was performed to investigate the potential impact of database origin on heterogeneity. Specifically, one Chinese literature article published in databases (Hu et al. 2017) was removed, and the remaining 10 English literature articles were re-evaluated. The study found that there were no significant changes in sensitivity (0.88 vs 0.89) and specificity (0.79 vs 0.79). The consistent results reinforce the robustness and reliability of the findings, providing a solid foundation for the stability and credibility of the conclusions drawn in this study.
The Publication bias and the influence of diagnostic results on disease prevalence probability
The Deeks funnel chart showed no evidence of publication bias, while the Fagan diagram illustrates that the probability of correctly predicting BI-RADS 4 benign and malignant breast lesions before using MRI radiomics is initially 50%. However, when MRI radiomics is used in the prediction process, the probability of predicting positive cases increases significantly to 81%, while the probability of predicting negative cases decreases to 13%. These results indicate that MRI radiomics improves the diagnostic accuracy of BI-RADS 4 benign and malignant breast lesions, highlighting the usefulness of this technique in clinical practice. Detailed illustrations of these findings are presented in the accompanying figure below (Fig. 6).
Discussion
Breast MRI shows promise as a modality for breast screening due to its superior soft tissue resolution and lack of radiation exposure. Studies have demonstrated its significant superiority over mammography and ultrasonography in terms of early breast cancer diagnosis and local staging (Thibault et al. 2013b; Hall-Beyer 2017). The BI-RADS (Rao et al. 2016) scoring system is a standardized way to assess the imaging characteristics of breast tumors and provide an approximate evaluation of lesion malignancy. The current classification system for breast cancer does not adequately account for the variations among tumors, resulting in the diverse biological behaviors observed. The NCCN guidelines recognize that BI-RADS 4 breast lesions have a malignancy probability ranging from 2 to 95%. However, the positive predictive value falls between 25.7% and 59.2% in reality (Vandenbroucke et al. 2014; Altman et al. 2001; Willinek and Schild 2008). Artificial intelligence has become increasingly prominent in the exploration and application of medical image data, as it overcomes the limitations associated with visual interpretation of tumor images (Lambin et al. 2017). It provides a noninvasive approach for predicting malignancy in breast lesions based on imaging data, offering diagnosticians and oncologists a reliable quantitative assessment tool.
This meta-analysis analyzed 11 studies that evaluated the effectiveness of MRI radiomics in detecting benign and malignant breast lesions classified as BI-RADS 4. The study included 1893 patients who were diagnosed with BI-RADS 4 breast lesions, which accounted for a total of 1915 lesions. The analysis results demonstrated that MRI radiomics exhibited high sensitivity and specificity in diagnosing BI-RADS 4 benign and malignant breast lesions. These findings highlight the potential of combining radiomics with MRI for the diagnosis of BI-RADS 4 lesions. Specifically, the study’s analysis indicated that the probability of accurately identifying true positive patients was 4.2 times greater than that of false positive patients (combined PLR = 4.2), resulting in a significant increase in the likelihood of detecting positive cases, while the probability of true negative patients was approximately 6.6 times higher than that of false negative patients (combined NLR = 0.15), indicating a substantial reduction in the likelihood of incorrectly identifying negative cases. These findings indicate that MRI-based radiomics exhibits substantial diagnostic efficacy in distinguishing between BI-RADS 4 benign and malignant breast lesions, underscoring its significant practical utility and wide-ranging applications.
The meta-analysis identified heterogeneity in the study, which could be attributed to various factors such as the ratio of individuals in the training set to the test set, contrast agents, tumor segmentation methods, feature selection algorithms, MRI equipment manufacturers, magnetic field strengths, and radiomics algorithms. It was concluded that proper allocation of individuals between the training and test sets is essential for the outcomes of machine learning and deep learning models (Song et al. 2020; Sedgwick 2013). Therefore, it is recommended to carefully determine an appropriate ratio to ensure reliable and robust results.
Notably, the unexpected finding of higher sensitivity in 1.5 T MRI as compared to 3.0 T MRI challenges the conventional understanding that higher magnetic fields enhance image resolution and diagnostic accuracy (Willinek and Schild 2008). However, this meta-analysis only included one study on 1.5 T MRI, indicating the necessity for further investigations with more 1.5 T MRI studies to confirm this observation.
The study found that manual segmentation has better sensitivity and specificity than automatic segmentation, potentially due to limitations in current automatic segmentation algorithms. Manual segmentation enables precise control through manual intervention, resulting in higher accuracy and comprehensiveness. Deep learning has exhibited higher sensitivity and specificity than machine learning in radiomics algorithms, but this observation is based on only three studies. Hence, further research is required to ensure the reliability and generalizability of these findings.
Subgroup analysis indicated that studies utilizing GE MRI equipment showed higher diagnostic performance compared to those using Siemens equipment, suggesting the impact of different MRI devices on diagnostic accuracy. Therefore, a prospective study is needed to compare the diagnostic performance of BI-RADS 4 breast benign and malignant lesions using these specific MRI devices based on MRI radiomics.
LASSO (Least Absolute Shrinkage and Selection Operator) is a commonly used feature selection technique in high-dimensional data analysis and regression (Ranstam et al. 2018). Previous research has shown that LASSO and logistic regression are commonly used in radiomics-related studies (Song et al. 2020). Our investigation found that LASSO has significant heterogeneity when compared with non-LASSO methods such as support vector machines, highlighting the need for further examination of the factors contributing to this heterogeneity. When using the LASSO method in future studies on diagnosing BI-RADS 4 breast lesions using MRI radiomics, it is important to carefully select an appropriate feature screening algorithm that aligns with the dataset’s characteristics to optimize the balance between sensitivity and specificity. This will enhance diagnostic accuracy and reliability.
In addition, it is important to consider the potential biases introduced by different contrast agents when using MRI radiomics for the diagnosis of BI-RADS 4 breast lesions. This requires an understanding of how the chemical structure of the contrast agent may impact the results. By taking these factors into account, we can improve our understanding and control of potential influencing factors, ultimately leading to more accurate and reliable outcomes.
To account for potential heterogeneity from the database source, we excluded the Chinese literature (Hu et al. 2017) from the 11 studies that were included. We then conducted a reanalysis using the remaining 10 studies that were in English. Our findings showed that there were no significant changes in sensitivity (0.88 vs. 0.89) and the degree of specificity (0.79 vs. 0.79).
The results of our study demonstrate stability and reliability. Nonetheless, it is crucial to recognize certain limitations. Firstly, it is important to note that all 11 studies analyzed were retrospective in nature, which means they lacked the rigorous data collection and control measures typically associated with prospective studies. Secondly, our meta-analysis revealed substantial heterogeneity in sensitivity (95% CI 0.83–0.92, I2 = 76.44%) and specificity (95% CI 0.72–0.84, I2 = 55.72%) due to variations in the statistical methods employed across different studies, encompassing model selection and effect size estimation methods. Despite this heterogeneity, the results of our study demonstrate stability and reliability. However, the literature reviewed in our study primarily focused on the effectiveness of MRI radiomics in diagnosing benign and malignant BI-RADS 4 breast lesions, but did not extensively evaluate specific subtypes within these categories. Future meta-analyses should consider including more studies that specifically target the assessment of subtypes within the benign and malignant BI-RADS 4 classifications. This would improve the comprehensiveness of the analysis and provide more valuable insights into the diagnostic capabilities of MRI radiomics for distinguishing between these subtypes.
Notably, in our study, 9 out of the 11 included studies utilized contrast-enhanced sequences, specifically Dynamic Contrast-Enhanced (DCE) sequences, for breast scanning. However, the role of other sequences in diagnosing BI-RADS 4 breast cancer, such as diffusion-weighted imaging (DWI), has been overlooked. This is a nonenhanced MRI technique that rapidly evaluates tissue characteristics by measuring the movement of water molecules within the tissues. In the context of breast cancer, restricted diffusion of water molecules is observed in comparison to normal breast tissue, resulting in a higher signal on DWI (Partridge et al. 2017). The recent studies have demonstrated the effectiveness of DWI-based radiomics in assessing the heterogeneity of breast tumors, providing valuable guidance for treatment decisions and noninvasive tumor monitoring (Leithner et al. 2020).Consequently, future research is expected to explore the potential of DWI radiomics in the evaluation of BI-RADS 4 benign and malignant breast lesions, presenting an exciting avenue for further investigation.
Conclusion
In conclusion, MRI-based radiomics has high diagnostic accuracy for BI-RADS 4 breast lesions. Further validation in large-scale studies will enhance patient outcomes by providing accurate diagnoses and guiding effective treatments.
Data availability
The datasets analyzed during the current study are available in the public databases PubMed, Embase, Cochrane Library, and Sinomed. The data from the 11 studies included can be traced and accessed via the DOIs provided in the references section of our manuscript.
References
Altman DG, Moher D, Schulz KF et al (2001) The Revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 134(8):663–694
Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM et al (2019) Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 381(22):2091–2102. https://doi.org/10.1056/NEJMoa1903986
Bennani-Baiti B, Dietzel M, Baltzer PA (2017) MRI for the assessment of malignancy in BI-RADS 4 mammographic microcalcifications. PLoS ONE 12(11):e0188679. https://doi.org/10.1371/journal.pone.0188679
Bickelhaupt S, Jaeger PF, Laun FB et al (2018) Radiomics based on adapted diffusion kurtosis imaging helps to clarify most mammographic findings suspicious for cancer. Radiology 287(3):761–770
Daimiel Naranjo I, Gibbs P, Reiner JS et al (2021) Radiomics and machine learning with multiparametric breast MRI for improved diagnostic accuracy in breast cancer diagnosis. Diagnostics 11(6):919
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58(9):882–893. https://doi.org/10.1016/j.jclinepi.2005.01.016
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188. https://doi.org/10.1016/0197-2456(86)90046-2
Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, Bezemer PD (2002) Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 3(2):9. https://doi.org/10.1186/1471-2288-2-9
Ellmann S, Wenkel E, Dietzel M et al (2020) Implementation of machine learning into clinical breast MRI: potential for objective and accurate decision-making in suspicious breast masses. PLoS One 15(1):e0228446
Goetz MP, Gradishar WJ, Anderson BO, Gradishar WJ, Anderson BO, Abraham J et al (2019) NCCN Guidelines Insights: Breast Cancer, Version 3.2018: featured updates to the NCCN Guidelines. J Natl Compr Cancer Net 17(2):118–26. https://doi.org/10.6004/jnccn.2019.0009
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A et al (2017) NCCN guidelines insights: breast cancer, version. J Natl Comprehens Cancer Network 15(4):433–451
Hall-Beyer M (2017) GLCM texture: a tutorial V. 3.0 March 2017. Calgary: University of Calgary Press. https://doi.org/10.11575/PRISM/33280
Hao W, Gong J, Wang S et al (2020) Application of MRI radiomics-based machine learning model to improve contralateral BI-RADS 4 lesion assessment[J]. Front Oncol 10:531476
Hu B, Xu K, Zhang L et al (2017) Apparent diffusion coefficient map based radiomics model in differentiating benign from malignant entity in breast imaging-reporting and data system 4 breast lesions. Chinese J Radiol 12:922–925
Hu Y, Cai Z, AiErken N J, et al (2023) Intra-and peri-tumoral radiomics based on dynamic contrast-enhanced MRI for prediction of benign disease in Bi-Rads 4 breast lesions: a multicentre study. Available at SSRN 4389618
Jonas R, Cook J (2018) LASSO regression. British J Surg 105(10):1348
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762. https://doi.org/10.1038/nrclinonc.2017.141
Leithner D, Bernard-Davila B, Martinez DF et al (2020) Radiomic signatures derived from diffusion-weighted imaging for the assessment of breast cancer receptor status and molecular subtypes. Mol Imag Biol 22:453–461
Liu Z, Wang S, Dong D, Wei J, Fang C, Zhou X et al (2019) The applications of radiomics in precision diagnosis and treatment of oncology: opportunities and challenges. Theranostics 9(5):1303. https://doi.org/10.7150/thno.30309
Lyu Y, Chen Y, Meng L et al (2023) Combination of ultrafast dynamic contrast-enhanced MRI-based radiomics and artificial neural network in assessing BI-RADS 4 breast lesions: Potential to avoid unnecessary biopsies. Front Oncol 13:107406
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM; and the PRISMA-DTA Group; Clifford T, Cohen JF, Deeks JJ, Gatsonis C, Hooft L, Hunt HA, Hyde CJ, Korevaar DA, Leeflang MMG, Macaskill P, Reitsma JB, Rodin R, Rutjes AWS, Salameh JP, Stevens A, Takwoingi Y, Tonelli M, Weeks L, Whiting P, Willis BH. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA Statement. JAMA. 2018;319(4):388–396. https://doi.org/10.1001/jama.2017.19163. Erratum in: JAMA. 2019;322(20):2026
Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE (2017) Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J Magn Reson Imaging 45(2):337–355
Rao AA, Feneis J, Lalonde C, Ojeda-Fournier H (2016) A pictorial review of changes in the BI-RADS Fifth Edition. Radiographics 36(3):623–39. https://doi.org/10.1148/rg.2016150178
Satake H, Ishigaki S, Ito R et al (2022) Radiomics in breast MRI: Current progress toward clinical application in the era of artificial intelligence. La Radiologia Medica. https://doi.org/10.1007/s11547-021-01423-y
Sedgwick P (2013) Prospective cohort studies: advantages and disadvantages. BMJ. https://doi.org/10.1136/bmj.f6726
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48
Sohn CK, Bisdas S (2020) Diagnostic accuracy of machine learning-based radiomics in grading gliomas: systematic review and meta-analysis. Contrast Media Mol Imag 2020(1–12):2127062. https://doi.org/10.1155/2020/2127062
Song J, Yin Y, Wang H et al (2020) A review of original articles published in the emerging field of radiomics. Eur J Radiol 127:108991
Thibault G, Fertil B, Navarro C, Pereira S, Cau P, Levy N et al (2013a) Shape and texture indexes application to cell nuclei classification. Int J Pattern Recognit Artif Intell 27(01):1357002. https://doi.org/10.1142/S0218001413570024
Thibault G, Angulo J, Meyer F (2013b) Advanced statistical matrices for texture characterization: application to cell classification. IEEE Trans Biomed Eng 61(3):630–637. https://doi.org/10.1109/TBME.2013.2284600
Vandenbroucke JP, von Elm E, Altman DG et al (2014) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int J Surg 12(12):1500–1524
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Willinek WA, Schild HH (2008) Clinical advantages of 3.0 T MRI over 1.5 T. Eur J Radiol 65(1):2–14
Ye DM, Wang HT, Yu T (2020) The application of radiomics in breast MRI: a review[J]. Technol Cancer Res Treat 19:1533033820916191
Yin H, Jiang Y, Xu Z et al (2022) Combined diagnosis of multiparametric MRI-based deep learning models facilitates differentiating triple-negative breast cancer from fibroadenoma magnetic resonance BI-RADS 4 lesions. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-022-04142-7
Zhang R, Wei W, Li R et al (2022) An MRI-based radiomics model for predicting the benignity and malignancy of BI-RADS 4 breast lesions[J]. Front Oncol 11:5541
Zhao YF, Chen Z, Zhang Y et al (2021) Diagnosis of breast cancer using radiomics models built based on dynamic contrast enhanced MRI combined with mammography. Front Oncol. https://doi.org/10.3389/fonc.2021.774248
Zhou J, Liu YL, Zhang Y et al (2021) BI-RADS reading of non-mass lesions on DCE-MRI and differential diagnosis performed by radiomics and deep learning. Front Oncol 11:728224
Acknowledgements
This study has received funding by two projects: ‘Construction of a deep convolutional neural network model for breast cancer screening based on large-sample conventional MR images’ (Project ID: LGF21H180003), which was funded by the Zhejiang Provincial Basic Public Welfare Research Program, and ‘Prediction of Traditional Chinese Medicine Subtypes in Breast Cancer based on Radiomic Features of Tumor Heterogeneity using High-resolution Diffusion-weighted Imaging (RESOLVE Sequence)’ (Project ID: 2022ZB132), which was funded by the Zhejiang Provincial Traditional Chinese Medicine Scientific Research
Funding
This study was funded by The Zhejiang Provincial Basic Public Welfare Research Program and The Zhejiang Provincial Traditional Chinese Medicine Scientific Research Program.
Author information
Authors and Affiliations
Contributions
Conception: GM, WJN, WSW; study design: LJ and ZH; data collection: LJ and ZH; data analysis: JQY and SJJ; data interpretation: JQY;manuscript writing: LJ and ZH; manuscript editing: All authors. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, J., Zheng, H., Jia, Q. et al. A meta-analysis of MRI radiomics-based diagnosis for BI-RADS 4 breast lesions. J Cancer Res Clin Oncol 150, 254 (2024). https://doi.org/10.1007/s00432-024-05697-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05697-3