Abstract
BRAF mutations are found in 1–5% of non-small-cell lung cancer (NSCLC), with V600 and non-V600 accounting for approximately 50% each. It has been confirmed that targeted therapy with dabrafenib + trametinib is effective in patients with metastatic NSCLC carrying BRAF V600E mutations. Preclinical studies have shown that dabrafenib + trametinib may also have inhibitory effects on some types of non-V600E mutations, especially some class II BRAF mutations. However, the efficacy of dabrafenib + trametinib on non-V600E mutant NSCLC in clinical practice only exists in some case reports. Here, we report a case of NSCLC patient carrying BRAF ex15 p.T599dup, who showed a clinical response to the combined therapy of dabrafenib + trametinib.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
B-RAF murine sarcoma viral oncogene homolog B1 (BRAF) is a proto-oncogene located on chromosome 7 (7q34), and is a serine/threonine protein kinase. BRAF participates in the mitogen-activated protein kinase (MAPK) cascade, which plays a crucial role in transmitting intracellular and extracellular signals, regulating cell proliferation, differentiation, and apoptosis (Patel et al. 2020). Mutations in the RAF gene phosphorylate mitogen-activated extracellular signal-regulated kinase (MEK) (downstream of the RAS–RAF–MEK–ERK pathway); the phosphorylated MEK causes sustained activation of extracellular regulated kinases (ERK), which ultimately leads to tumorigenesis. All RAF proteins have the ability to phosphorylate MEK, but BRAF is the most active (Śmiech et al. 2020).
The prevalence of BRAF mutations is 3.9% in all malignancies, with melanoma (39.7%), thyroid cancer (33.3%) and small bowel malignancies (8.9%) having the highest prevalence of mutations (Owsley et al. 2021). The incidence of BRAF mutations ranges from 1 to 5% in non-small-cell lung cancer (NSCLC), and is most common in lung adenocarcinomas, where BRAF V600E mutations account for more than 50% of all the BRAF mutation cases (O'Leary et al. 2019). Based on the BRF113928 study, the US Food and Drug Administration (FDA) approved the indication for the combination of dabrafenib (BRAF inhibitor) + trametinib (MEK inhibitor) for the treatment of advanced NSCLC patients carrying the BRAF V600E mutation in December 2017 (Odogwu et al. 2018). The incidence of non-V600E in BRAF mutations is nearly 50%, but the treatment strategy for non-V600E mutations remains limited. Currently, only some cases have reported the efficacy of RAF/MEK inhibitors in NSCLC patients with non-V600E mutations. Here, we report a case of a patient with a rare mutation in BRAF who showed a clinical response to the combination therapy of dabrafenib + trametinib.
Case presentation
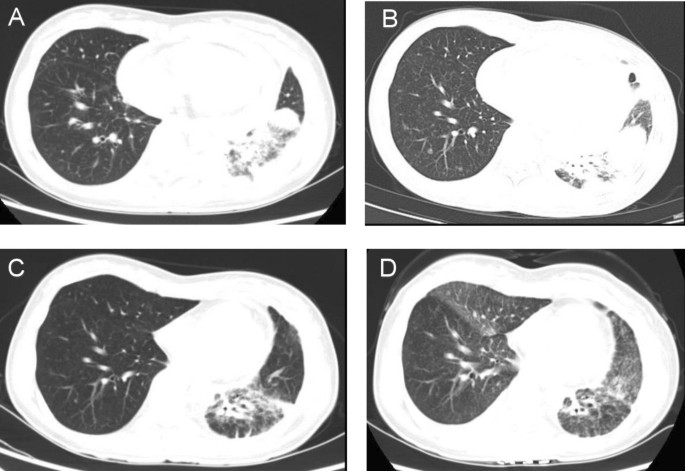
The patient was a 34-year-old female without a history of smoking who presented with cough and short of breath in 2021. A chest ultrasound indicated a large amount of fluid in the left pleural cavity and a small amount of fluid in the pericardium, so thoracentesis and pleural biopsy were performed. Pleural biopsy pathology suggested adenocarcinoma, immunohistochemistry showed positive TTF and negative P63. Positron emission tomography/computer tomography (PET/CT) showed a large lingual segment of the upper lobe of the left lung and a solid lesion in the lower lobe of the left lung with air bronchial signs, increased metabolism of the solid lung tissue, localized thickening of the pleura bilaterally, bilateral pleural effusion, a small amount of pericardial effusion, and an elevated SUV value (Fig. 1A). Next-generation sequencing showed BRAF exon 15 p.T599dup, TP53 exon 8 p.V272M missense mutation, BRCA2 exon 11 missense mutation andCTNNB1 exon 3 missense mutation. The patient was finally diagnosed as left lung adenocarcinoma with left pleural and pericardial metastases (cT3N0M1a, stage IVA, BRAF ex15 p.T599dup mutation positive).
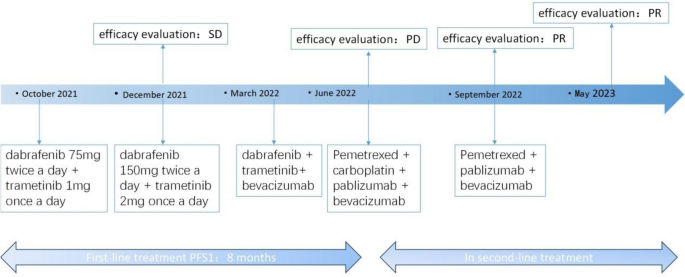
BRAF ex15 p.T599dup belongs to class II BRAF mutation. Only one case report indicated that a patient of NSCLC with a BRAF ex15 p.T599dup mutation exhibited a durable response to the combination therapy of dabrafenib + trametinib (Turshudzhyan and Vredenburgh 2020). Considering that the patient has pericardial effusion with symptom of short of breath and low physical status (PS)score of 3, the patient decided to receive combination therapy of dabrafenib + trametinib, for the reason that time to response of targeted therapy is much faster than chemotherapy or immunotherapy. The patient was initially treated with dabrafenib and trametinib with prior patient’s consent in reduced doses (dabrafenib 75 mg twice a day and trametinib 1 mg once a day) due to a PS score of 3 (Fig. 2). The patient's adverse event during treatment was fever with a maximum temperature of 40 degrees Celsius, which returned to normal after antipyretic treatment with ibuprofen. After one month of targeted therapy, the patient’s symptoms of cough and short of breath were significantly improved, and the PS score was increased to 1. The efficacy evaluation was assessed as stable disease (SD), so the dose was adjusted to dabrafenib 150 mg twice a day and trametinib 2 mg once a day. After three months of treatment, the patient presented with symptoms of cardiac fatigue and dyspnea following physical activity, and cardiac ultrasound showed a large amount of pericardial effusion, and pericardiocentesis was performed to drain the pericardium. The morphological features of pericardial cytology suggested adenocarcinoma cells. Considering slow progression of the lung cancer with the intrapulmonary lesion did not display immediate enlargement bevacizumab was added to the targeted therapy as anti-vascular therapy. After eight months of treatment, the patient's symptoms such as cough and short of breath were worse than before, chest computed tomography (CT) scan showed new nodules in both lungs (Fig. 1B), and pericardial effusion increased than before, so the efficacy was evaluated as progression disease (PD) with 8 months of the progression-free survival 1 (PFS1). The patient’s PS score was 1 and PD-L1 expression was 5%, so pemetrexed + carboplatin + pembrolizumab + bevacizumab was chosen as patient’s second-line treatment regimen, and the best efficacy evaluation was partial response (PR) during the course of second-line treatment (Fig. 1C). After four cycles of treatment, the patient was treated with pemetrexed + pembrolizumab + bevacizumab for maintenance treatment, adverse events were 1st degree myelosuppression and gastrointestinal reactions. The patient is now on second-line treatment for 1 year and the efficacy evaluation is still in continuous PR (Fig. 1D).
Discussion
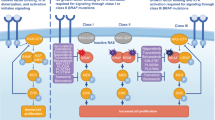
BRAF is a component of the RAS–RAF–MEK–ERK pathway, and mutations in the BRAF gene phosphorylate MEK, which leads to sustained downstream activation and finally to tumorigenesis. BRAF mutations can be classified into the following three categories based on three aspects: whether the mutation has kinase activity, whether the kinase activity is dependent on RAS activation, and whether dimerization is required. Class I: is not dependent on upstream RAS signaling and exhibits low RAS activity due to the negative feedback of the RAS–RAF–MEK–ERK pathway. For wild-type BRAF, the formation of a BRAF dimer is required to activate the downstream pathway; whereas in class I mutations, BRAF acts as a constitutively active monomer and does not require a dimer to activate the downstream EKR pathway. The most common is the V600E mutation, in which glutamate (E) replaces valine (V) at the 600th site. Class II: Like class 1 mutations, they are also not dependent on upstream RAS signaling but require RAF dimer formation to activate the downstream EKR pathway. The location of class II mutations (activation fragment or P-loop) can block the pathway's self-inhibitory mechanism and thus maintain high kinase activity. Class II mutations include K601, L597, G464, G469, etc., and the rare mutation BRAF ex15 p.T599dup reported in this case also belongs to class II. Class III: Including G466, N581, D594, G596, etc., belonging to the kinase-impaired non-V600 mutant heterodimers with high RAS-GTP levels, which require upstream activation and transmit activation signals downstream by forming a dimer with wild-type CRAF, mutations at the DFG motif lead to impaired kinase activity (Frisone et al. 2020; Śmiech et al. 2020).
Research on targeted therapy for BRAF has focused on the V600E mutation, but class II and III BRAF mutations have also been found to be the driving mutations in lung adenocarcinoma, and the rate of non-BRAF V600E mutations can be as high as 50–80% in all BRAF mutant lung cancers (Bracht et al. 2019). Therefore, research on targeted therapy for non-BRAF V600E mutations is a breakthrough point to solve the clinical dilemma of BRAF mutation treatment. Currently, the FDA has approved three BRAF inhibitors to be used alone or in combination with other drugs in patients with BRAF V600E mutations, and the combination of dabrafenib + trametinib has been approved for patients with BRAF V600E mutations in advanced NSCLC (Poulikakos et al. 2022). In terms of the mechanism of BRAF class II and III mutations, they are all potential beneficiaries of combination therapy with RAF/MEK inhibitors, but the efficacy of combination therapy with RAF/MEK inhibitors for non-BRAF V600E mutations is currently uncertain.
To the best of our knowledge, this is the 2nd reported case of a patient with NSCLC carrying BRAF ex15 p.T599dup benefit from the combination therapy of dabrafenib + trametinib. The first patient was a 75-year-old woman diagnosed with NSCLC, a non-smoker with a history of breast cancer, reported by Alla Turshudzhyan et al. The tumor regressed significantly after 4 months of dabrafenib + trametinib treatment (Turshudzhyan and Vredenburgh 2020). Similarly, the patient in our study responded quickly from dabrafenib + trametinib treatment. After one month of treatment, the patient’s symptoms of cough and short of breath were significantly improved, and the PS score was increased from 3 to 1. One of the advantages for targeted therapy is the rapid onset of action, their time to response is much faster than chemotherapy or immunotherapy. Thus, for the patients with low PS score and need to improve symptoms quickly, targeted therapy may be a good choice. Moreover, the combination of bevacizumab and dabrafenib + trametinib can not only improve pericardial effusion, but also delaying drug resistance. After the recurrence of pericardial effusion, adding bevacizumab to dabrafenib + trametinib can make patient still benefit from targeted therapy for another 3 months.
Preclinical studies have shown that trametinib in combination with or without dabrafenib effectively inhibits cell growth in the H2087 (L597V) and H1755 (G469A) lung cancer cell lines. However, even patients with the same BRAF class II mutation may respond differently to the treatment with dabrafenib + trametinib. Patients carrying the BRAF L597R mutation were sensitive to trametinib + dabrafenib, with significant clinical benefit and sustained response for one year. Whereas patients carrying the G469V mutation were resistant to trametinib + dabrafenib, patients experienced rapid disease progression within 2 months. This may be due to the fact that patients carry a more complex gene profile (APC R1040fs∗16, CHD2 L1383∗, NFKBIA amplification, NKX2-1 amplification) (Negrao et al. 2020). Due to the diversity and functional heterogeneity of non-BRAF V600E mutations, certain locus-specific non-BRAF V600E mutations may be effective in the treatment of dabrafenib + trametinib, but only a few case reports are available at present (Reyes et al. 2019; Su et al. 2021; Turshudzhyan and Vredenburgh 2020). Both clinical and preclinical data suggest that targeted therapy with BRAF and MEK inhibitors is feasible in patients with some types of non-V600E mutations. More future studies are needed to validate the efficacy of targeted therapy in patients with this rare mutation.
Conclusion
We report a case of a lung adenocarcinoma patient with a rare BRAF mutation who benefited from the combination therapy of dabrafenib + trametinib. We hope to draw attention to patients carrying BRAF non-V600E mutations and explore the best treatment strategy in the future.
References
Bracht J, Karachaliou N, Bivona T, Lanman RB, Faull I, Nagy RJ et al (2019) BRAF mutations classes I, II, and III in NSCLC patients included in the SLLIP trial: the need for a new pre-clinical treatment rationale. Cancers (basel) 11(9):1381. https://doi.org/10.3390/cancers11091381
Frisone D, Friedlaender A, Malapelle U, Banna G, Addeo A (2020) A BRAF new world. Crit Rev Oncol Hematol 152:103008. https://doi.org/10.1016/j.critrevonc.2020.103008
Negrao MV, Raymond VM, Lanman RB, Robichaux JP, He J, Nilsson MB et al (2020) Molecular landscape of BRAF-mutant NSCLC reveals an association between clonality and driver mutations and identifies targetable non-V600 driver mutations. J Thorac Oncol 15(10):1611–1623. https://doi.org/10.1016/j.jtho.2020.05.021
Odogwu L, Mathieu L, Blumenthal G, Larkins E, Goldberg KB, Griffin N et al (2018) FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring BRAF V600E mutations. Oncologist 23(6):740–745. https://doi.org/10.1634/theoncologist.2017-0642
O’Leary CG, Andelkovic V, Ladwa R, Pavlakis N, Zhou C, Hirsch F et al (2019) Targeting BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res 8(6):1119–1124. https://doi.org/10.21037/tlcr.2019.10.22
Owsley J, Stein MK, Porter J, In GK, Salem M, O’Day S et al (2021) Prevalence of class I-III BRAF mutations among 114,662 cancer patients in a large genomic database. Exp Biol Med (Maywood) 246(1):31–39. https://doi.org/10.1177/1535370220959657
Patel H, Yacoub N, Mishra R, White A, Long Y, Alanazi S et al (2020) Current advances in the treatment of BRAF-mutant melanoma. Cancers (basel) 12(2):482. https://doi.org/10.3390/cancers12020482
Poulikakos PI, Sullivan RJ, Yaeger R (2022) Molecular pathways and mechanisms of BRAF in cancer therapy. Clin Cancer Res 28(21):4618–4628. https://doi.org/10.1158/1078-0432.CCR-21-2138
Reyes R, Mayo-de-Las-Casas C, Teixidó C, Cabrera C, Marín E, Vollmer I et al (2019) Clinical benefit from BRAF/MEK inhibition in a double non-V600E BRAF mutant lung adenocarcinoma: a case report. Clin Lung Cancer 20(3):e219–e223. https://doi.org/10.1016/j.cllc.2019.02.022
Śmiech M, Leszczyński P, Kono H, Wardell C, Taniguchi H (2020) Emerging BRAF mutations in cancer progression and their possible effects on transcriptional networks. Genes (basel) 11(11):1342. https://doi.org/10.3390/genes11111342
Su PL, Lin CY, Chen YL, Chen WL, Lin CC, Su WC (2021) Durable response to combined dabrafenib and trametinib in a patient with BRAF K601E mutation-positive lung adenocarcinoma: a case report. JTO Clin Res Rep 2(8):100202. https://doi.org/10.1016/j.jtocrr.2021.100202
Turshudzhyan A, Vredenburgh J (2020) A rare p.T599dup BRAF mutant NSCLC in a non-smoker. Curr Oncol 28(1):196–202. https://doi.org/10.3390/curroncol28010021
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors listed in this manuscript contributed significantly to the study. LJ contributed to write the manuscript, major revision of the manuscript and took the whole responsibility of literature search. PRY and YFL contributed to the data acquisition. JL contributed to reviewing the manuscript for critical revisions and full access to the data. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, L., Yang, P., Liu, Y. et al. BRAF/MEK-targeted therapy in BRAF ex15 p.T599dup mutation-driven NSCLC: a case report. J Cancer Res Clin Oncol 150, 162 (2024). https://doi.org/10.1007/s00432-024-05675-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05675-9