Abstract
Purpose
Drug resistance inevitably occurs despite the encouraging results of immunotherapy. This study attempted to investigate immunotherapy rechallenge treatment regimens and factors associated with outcomes in patients with non-small cell lung cancer (NSCLC) according to resistance status.
Methods
A retrospective study was conducted on patients with advanced NSCLC who received immune checkpoint inhibitor (ICI) monotherapy and immune rechallenge between March 2016 and December 2022. Primary resistance (RR) was defined by an absence of response after treatment administered for less than 6 months before progression. Acquired resistance (AR) was defined as a response to immunotherapy treatment administered for more than 6 months before progression. Disease progression in as many as three lesions was defined as systemic progression, whereas disease progression in fewer than three lesions was defined as oligo-progression.
Results
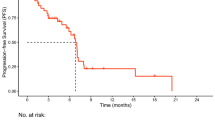
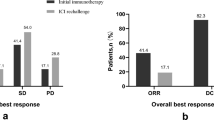
Of 40 patients, 18 (45%) had primary resistance, and 22 (55%) developed AR. Overall survival (OS) was not reached. A significant difference in progression-free survival (PFS) was observed in individuals rechallenged with ICIs after AR and RR (7.0 months vs. 2.1 months, P = 0.003). Patients receiving interval treatment before rechallenge achieved longer PFS than those who did not (6.2 months vs. 4.0 months, P = 0.027). Multivariate analysis demonstrated that systemic progression was a risk factor significantly associated with PFS after ICI rechallenge (P = 0.006). After AR, ICI rechallenge prolonged the duration of PFS if patients developed oligo-progression (5.4 months vs. 1.1 months, P < 0.001).
Conclusion
ICI rechallenge is likely to be an option for patients with oligo-progression during rechallenge, particularly after AR.



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Brahmer JR, Lee JS, Ciuleanu TE, Bernabe Caro R, Nishio M, Urban L, Audigier-Valette C, Lupinacci L, Sangha R, Pluzanski A, Burgers J, Mahave M, Ahmed S, Schoenfeld AJ, Paz-Ares LG, Reck M, Borghaei H, O’Byrne KJ, Gupta RG, Bushong J, Li L, Blum SI, Eccles LJ, Ramalingam SS (2023) Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J Clin Oncol 41(6):1200–1212. https://doi.org/10.1200/JCO.22.01503
Chang J, Wu YL, Lu S, Wang J, Mok T, Zhang L, Feng J, Wu L, Tu HY, Zhang Y, Luft A, Zhou JY, Ma Z, Lu Y, Hu C, Shi Y, Poddubskaya E, Soo RA, Chia YH, Penrod JR, Taylor F, Lawrance R, Blum SI, Sun X, Juarez-Garcia A, Moreno-Koehler A, Li A, Li A, Cheng Y (2021) Three-year follow-up and patient-reported outcomes from CheckMate 078: nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non-small cell lung cancer. Lung Cancer 165:71–81. https://doi.org/10.1016/j.lungcan.2021.12.009
Chen P, Liu Y, Wen Y, Zhou C (2022) Non-small cell lung cancer in China. Cancer Commun (lond) 42(10):937–970. https://doi.org/10.1002/cac2.12359
Chu RW, Vegas García A, Hickey C, Power DG, Gorry C (2022) Cost-effectiveness of first-line pembrolizumab monotherapy versus chemotherapy in high programmed death-ligand 1 advanced non-small cell lung cancer in the Irish healthcare setting. Value Health 26(3):402–410. https://doi.org/10.1016/j.jval.2022.10.012
Fujita K, Yamamoto Y, Kanai O, Okamura M, Hashimoto M, Nakatani K, Sawai S, Mio T (2020) Retreatment with anti-PD-1 antibody in non-small cell lung cancer patients previously treated with anti-PD-L1 antibody. Thorac Cancer 11(1):15–18. https://doi.org/10.1111/1759-7714.13241
Gettinger SN, Wurtz A, Goldberg SB, Rimm D, Schalper K, Kaech S, Kavathas P, Chiang A, Lilenbaum R, Zelterman D, Politi K, Herbst RS (2018) Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non-small cell lung cancer. J Thorac Oncol 13(6):831–839. https://doi.org/10.1016/j.jtho.2018.03.008
Giaj Levra M, Cotté FE, Corre R, Calvet C, Gaudin AF, Penrod JR, Grumberg V, Jouaneton B, Jolivel R, Assié JB, Chouaïd C (2020) Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer 140:99–106. https://doi.org/10.1016/j.lungcan.2019.12.017
Guo M, VanderWalde AM, Yu X, Vidal GA, Tian GG (2022) Immune checkpoint inhibitor rechallenge safety and efficacy in stage IV non-small cell lung cancer patients after immune-related adverse events. Clin Lung Cancer 23(8):686–693. https://doi.org/10.1016/j.cllc.2022.07.015
Halmos B, Burke T, Kalyvas C, Vandormael K, Frederickson A, Piperdi B (2021) Pembrolizumab+chemotherapy versus atezolizumab+chemotherapy+/− bevacizumab for the first-line treatment of non-squamous NSCLC: a matching-adjusted indirect comparison. Lung Cancer 155:175–182. https://doi.org/10.1016/j.lungcan.2021.03.020
Hirano S, Hayama N, Tabeta H, Kuroki T, Shiraishi Y, Fujita T, Amano H, Nakamura M, Shimizu S, Nakamura S (2020) Drastic response of rechallenge of nivolumab in a patient with NSCLC who progressed on the first nivolumab treatment. J Thorac Oncol 15(1):e20–e22. https://doi.org/10.1016/j.jtho.2019.10.012
Hosoya K, Fujimoto D, Morimoto T, Kumagai T, Tamiya A, Taniguchi Y, Yokoyama T, Ishida T, Matsumoto H, Hirano K, Kominami R, Tomii K, Suzuki H, Hirashima T, Tanaka S, Uchida J, Morita M, Kanazu M, Mori M, Nagata K, Fukuda I, Tamiya M (2021) Clinical factors associated with shorter durable response, and patterns of acquired resistance to first-line pembrolizumab monotherapy in PD-L1-positive non-small-cell lung cancer patients: a retrospective multicenter study. BMC Cancer 21(1):346. https://doi.org/10.1186/s12885-021-08048-4
Kluger HM, Tawbi HA, Ascierto ML, Bowden M, Callahan MK, Cha E, Chen HX, Drake CG, Feltquate DM, Ferris RL, Gulley JL, Gupta S, Humphrey RW, LaVallee TM, Le DT, Hubbard-Lucey VM, Papadimitrakopoulou VA, Postow MA, Rubin EH, Sharon E, Taube JM, Topalian SL, Zappasodi R, Sznol M, Sullivan RJ (2020) Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC immunotherapy resistance taskforce. J Immunother Cancer 8(1):e000398. https://doi.org/10.1136/jitc-2019-000398
Nagasaki J, Ishino T, Togashi Y (2022) Mechanisms of resistance to immune checkpoint inhibitors. Cancer Sci 113(10):3303–3312. https://doi.org/10.1111/cas.15497
Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee KH, Golf A, Sugawara S, Robinson AG, Halmos B, Jensen E, Schwarzenberger P, Pietanza MC, Paz-Ares L (2023) Pembrolizumab plus chemotherapy in squamous non-Small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol 41(11):1999–2006. https://doi.org/10.1200/JCO.22.01990
Schoenfeld AJ, Rizvi HA, Memon D, Shaverdian N, Bott MJ, Sauter JL, Tsai CJ, Lihm J, Hoyos D, Plodkowski AJ, Perez-Johnston R, Sawan P, Egger JV, Greenbaum BD, Rimner A, Riely GJ, Rudin CM, Rusch VW, Gomez DR, Hellmann MD (2022) Systemic and oligo-acquired resistance to PD-(L)1 blockade in lung cancer. Clin Cancer Res 28(17):3797–3803. https://doi.org/10.1158/1078-0432.CCR-22-0657
Shah S, Wood K, Labadie B, Won B, Brisson R, Karrison T, Hensing T, Kozloff M, Bao R, Patel JD, Luke JJ (2018) Clinical and molecular features of innate and acquired resistance to anti-PD-1/PD-L1 therapy in lung cancer. Oncotarget 9(4):4375–4384. https://doi.org/10.18632/oncotarget.23315
Siegel RL, Miller KD, Fuchs HE, Jemel A (2022) Cancer statistics. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Takahara Y, Tanaka T, Ishige Y, Shionoya I, Yamamura K, Sakuma T, Nishiki K, Nakase K, Nojiri M, Kato R, Shinomiya S, Fujimoto Y, Oikawa T, Mizuno S (2022) Efficacy and predictors of rechallenge with immune checkpoint inhibitors in non-small cell lung cancer. Thorac Cancer 13(4):624–630. https://doi.org/10.1111/1759-7714.14309
Vesely MD, Zhang T, Chen L (2022) Resistance mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol 40(1):45–74. https://doi.org/10.1146/annurev-immunol-070621-030155
Xu Z, Hao X, Yang K, Wang Q, Wang J, Lin L, Teng F, Li J, Xing P (2022) Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study. J Cancer Res Clin Oncol 148(11):3081–3089. https://doi.org/10.1007/s00432-021-03901-2
Acknowledgements
The authors appreciate all patients for their cooperation and participation. In addition, we are thankful to all research staff and co-investigators involved in this study.
Funding
The study was supported by the Medical Scientific Research Foundation of Zhejiang Province (No.2022KY653), and sponsored by Zhejiang provincial program for the Cultivation of High-Level Innovative Health Talents (to Zhengbo Song).
Author information
Authors and Affiliations
Contributions
ZS designed and supervised the research. MX, YH and ZS conducted the follow-up, data collection and correlative analysis. ZS provided support in data analysis and use of software. MX and YH provided data analysis. All authors were involved in manuscript preparation and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethics approval and consent to participate
Approval of the study protocol was obtained from Zhejiang Cancer Hospital Institutional Review Board Committee (approval number: IRB-2022-187). Individual consent for this retrospective analysis was waived.
Consent for publication
Not applicable.
Consent to participate and publish
Individual consent for analysis was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2023_5490_MOESM1_ESM.pdf
Supplementary file1 Supplementary Fig.1 Kaplan–Meier curves of PFS for the initial immunotherapy of the entire group. The median PFS of patients in the entire group was 5.2 (95%CI: 3.6 to 6.8) months when treated with the initial immune monotherapy (PDF 4 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, M., Hao, Y., Shi, Z. et al. Efficacy of rechallenge immunotherapy after immune monotherapy resistance in patients with advanced non-small cell lung cancer. J Cancer Res Clin Oncol 149, 17987–17995 (2023). https://doi.org/10.1007/s00432-023-05490-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05490-8