Abstract
Background
Chimeric antigen receptor (CAR) T cell therapy has shown promising results in treating blood cancers, but it has limited efficacy against solid tumors that express mesothelin (MSLN). One of the reasons is the immunosuppressive tumor microenvironment, which consists of physical barriers, multiple mechanisms of immune evasion, and various biochemical factors that favor tumor growth and survival. These factors reduce the antitumor activity of MSLN-targeted CAR T cells in clinical trials. Therefore, new therapeutic strategies are needed to enhance the effectiveness of MSLN-targeted CAR T cell therapy.
Methods
To investigate whether the antitumor efficacy of anti-MSLN CAR-T cells depends on the epitopes they recognize, we generated MSLN-targeted CAR T cells that bind to different regions of MSLN (Region I, II, III and Full length). We then evaluated the antitumor activity of MSLN-targeted CAR T cells alone or in combination with the chemotherapeutic drug irinotecan or an anti-PD-1 antibody in vitro and in vivo.
Results
We found that MSLN-targeted CAR T cells effectively killed MSLN-positive cancer cells (H9, H226 and Panc-1), but not MSLN-negative cells (A431) in vitro. In a mouse model of H9 tumor xenografts, all CAR T cells showed similar tumor suppression, but an MSLN-targeted scFv with Region I epitope, R47, performed slightly better. Combining irinotecan with CAR_R47 T cells enhanced tumor control synergistically in both H9 xenograft mice and patient-derived xenograft mice.
Conclusions
Our results suggest that irinotecan can enhance the antitumor activity of MSLN-targeted CAR T cells, and offer a promising combination therapy strategy for MSLN-positive solid tumors.





Similar content being viewed by others
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Adusumilli PS, Zauderer MG, Riviere I, Solomon SB, Rusch VW, O’Cearbhaill RE, Zhu A, Cheema W, Chintala NK, Halton E et al (2021) A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov 11:2748–2763
Ager A, Watson HA, Wehenkel SC, Mohammed RN (2016) Homing to solid cancers: a vascular checkpoint in adoptive cell therapy using CAR T-cells. Biochem Soc Trans 44:377–385
Beatty GL, O’Hara M (2016) Chimeric antigen receptor-modified T cells for the treatment of solid tumors: defining the challenges and next steps. Pharmacol Ther 166:30–39
Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM et al (2014) Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2:112–120
Beatty GL, O’Hara MH, Lacey SF, Torigian DA, Nazimuddin F, Chen F, Kulikovskaya IM, Soulen MC, McGarvey M, Nelson AM et al (2018) Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155:29–32
Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M et al (2013) CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5:177ra138
Chang K, Pastan I (1996) Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA 93:136–140
Chen X, Amar N, Zhu Y, Wang C, Xia C, Yang X, Wu D, Feng M (2020) Combined DLL3-targeted bispecific antibody with PD-1 inhibition is efficient to suppress small cell lung cancer growth. J Immunother Cancer 8:e000785
Constantinidou A, Alifieris C, Trafalis DT (2019) Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther 194:84–106
Danz M, Pottgen K, Tonjes PM, Hinkelbein J, Braunecker S (2016) Hyponatremia among triathletes in the ironman European Championship. N Engl J Med 374:997–998
Feng M, Gao W, Wang R, Chen W, Man YG, Figg WD, Wang XW, Dimitrov DS, Ho M (2013) Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci USA 110:E1083-1091
Gray KD, McCloskey JE, Vedvyas Y, Kalloo OR, Eshaky SE, Yang Y, Shevlin E, Zaman M, Ullmann TM, Liang H et al (2020) PD1 blockade enhances ICAM1-directed CAR T therapeutic efficacy in advanced thyroid cancer. Clin Cancer Res 26:6003–6016
Guo Y, Wang Y, Han W (2016) Chimeric antigen receptor-modified T cells for solid tumors: challenges and prospects. J Immunol Res 2016:3850839
Haas AR, Tanyi JL, O’Hara MH, Gladney WL, Lacey SF, Torigian DA, Soulen MC, Tian L, McGarvey M, Nelson AM et al (2019) Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol Ther 27:1919–1929
Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D (2005) Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol 124:838–845
Hassan R, Tomar S, Zhang J, Khanal M, Hong J, Venugopalan A, Jiang Q, Sengupta M, Miettinen M, Li N et al (2022) Development of highly effective anti-mesothelin hYP218 chimeric antigen receptor T cells with increased tumor infiltration and persistence for treating solid tumors. Mol Cancer Ther 21:1195–1206
Jiang H, Song B, Wang P, Shi B, Li Q, Fan M, Di S, Yang J, Li Z (2017) Efficient growth suppression in pancreatic cancer PDX model by fully human anti-mesothelin CAR-T cells. Protein Cell 8:926–931
June C, O’Connor R, Kawalekar O, Ghassemi S, Milone M (2018) CAR T cell immunotherapy for human cancer. Science (new York, NY) 359:1361–1365
Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, Maus MV, Fraietta JA, Zhao Y, June CH (2018) Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther 26:1855–1866
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM et al (2013) Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122:4129–4139
Liu G, Zhang Q, Li D, Zhang L, Gu Z, Liu J, Liu G, Yang M, Gu J, Cui X et al (2021) PD-1 silencing improves anti-tumor activities of human mesothelin-targeted CAR T cells. Hum Immunol 82:130–138
Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR et al (2015) 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 21:581–590
Long AH, Highfill SL, Cui Y, Smith JP, Walker AJ, Ramakrishna S, El-Etriby R, Galli S, Tsokos MG, Orentas RJ, Mackall CL (2016) Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol Res 4:869–880
Long KB, Young RM, Boesteanu AC, Davis MM, Melenhorst JJ, Lacey SF, DeGaramo DA, Levine BL, Fraietta JA (2018) CAR T cell therapy of non-hematopoietic malignancies: detours on the road to clinical success. Front Immunol 9:2740
Moon EK, Wang L-C, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Puré E, Milone MC et al (2014) Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 20:4262–4273
Morello A, Sadelain M, Adusumilli PS (2016) Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov 6:133–146
Newick K, O’Brien S, Moon E, Albelda SM (2017) CAR T cell therapy for solid tumors. Annu Rev Med 68:139–152
Ordonez NG (2003) Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol 16:192–197
O’Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A et al (2017) A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med 9:399
Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P et al (2018) Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 36:847–856
Roth TL, Li PJ, Blaeschke F, Nies JF, Apathy R, Mowery C, Yu R, Nguyen MLT, Lee Y, Truong A et al (2020) Pooled knockin targeting for genome engineering of cellular immunotherapies. Cell 181(728–744):e721
Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z et al (2011) CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Investig 121:1822–1826
Schmidts A, Maus MV (2018) Making CAR T cells a solid option for solid tumors. Front Immunol 9:2593
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jager U, Jaglowski S, Andreadis C, Westin JR et al (2019) Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380:45–56
Sotoudeh M, Shirvani SI, Merat S, Ahmadbeigi N, Naderi M (2019) MSLN (Mesothelin), ANTXR1 (TEM8), and MUC3A are the potent antigenic targets for CAR T cell therapy of gastric adenocarcinoma. J Cell Biochem 120:5010–5017
Tang H, Qiao J, Fu YX (2016) Immunotherapy and tumor microenvironment. Cancer Lett 370:85–90
Tang N, Cheng C, Zhang X, Qiao M, Li N, Mu W, Wei XF, Han W, Wang H (2020) TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. https://doi.org/10.1172/jci.insight.133977
van der Stegen SJ, Hamieh M, Sadelain M (2015) The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 14:499–509
Verma A, Rafiq S (2022) Chimeric antigen receptor (CAR) T cell therapy for glioblastoma. Cancer Treat Res 183:161–184
Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahmed R, Freeman G, Krogsgaard M, Riley J (2013) Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA 110:E2480-2489
Zhang YF, Phung Y, Gao W, Kawa S, Hassan R, Pastan I, Ho M (2015) New high affinity monoclonal antibodies recognize non-overlapping epitopes on mesothelin for monitoring and treating mesothelioma. Sci Rep 5:9928
Zhang Z, Jiang D, Yang H, He Z, Liu X, Qin W, Li L, Wang C, Li Y, Li H et al (2019) Modified CAR T cells targeting membrane-proximal epitope of mesothelin enhances the antitumor function against large solid tumor. Cell Death Dis 10:476
Funding
This work was supported by Natural Science Foundation of Hubei Province (2021CFB155), China Postdoctoral Science Foundation (2021M701338), the National Natural Science Foundation of China (32270992). Part of the work was supported by Postdoctoral Creative Research Positions of Hubei Province of China (2021) and the Baichuan Project at the College of Life Science and Technology, Huazhong Agricultural University.
Author information
Authors and Affiliations
Contributions
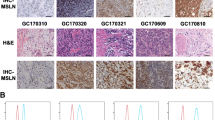
YZ performed the experiments, analyzed the data, prepared the figures, and drafted the manuscript. DZ, KW and SL carried out the flowcytometry and the animal studies. HH and LC participated in sample collection and immunohistochemistry. CX did PDX experiments and analyzed the data. MF designed the project and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethics approval and consent to participate
All the procedures used in the animal studies were approved by the Animal Care and Use Committee (ACUC) of Huazhong Agricultural University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Zuo, D., Wang, K. et al. Mesothelin-targeted CAR-T therapy combined with irinotecan for the treatment of solid cancer. J Cancer Res Clin Oncol 149, 15027–15038 (2023). https://doi.org/10.1007/s00432-023-05279-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05279-9