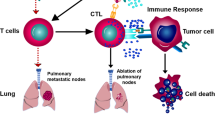
Abstract
Purpose
In a previous study, protein tyrosine phosphatase non-receptor type (PTPN) 3 was identified as an immune checkpoint molecule in lymphocytes, and its potential as a novel target for cancer immunotherapy was anticipated. However, evaluation of dendritic cell (DC) function as antigen-presenting cells is critical for the development of immunotherapy. In this study, we aimed to analyze the biological effect of PTPN3 on DCs induced from human peripheral blood monocytes obtained from healthy individuals.
Methods
We used short-interfering RNA to knock down PTP3 in DCs. For DC maturation, we added cancer cell lysate and tumor necrosis factor-α/interferon-α to immature DCs. In the cytotoxic assay, the target cancer cells were SBC5, unmatched with DCs from healthy human leukocyte antigen (HLA)-A24, or Sq-1, matched with DCs. Enzyme-linked immunosorbent assay was used to determine the amount of cytokines. To examine the intracellular signaling system, intracellular staining was used.
Results
PTPN3 knockdown significantly increased the number of DCs, expression of CD80 and chemokine receptor (CCR)7, and production of interleukin-12p40/p70 in mature DCs. In the HLA-A24-restricted DC and human lung squamous cell carcinoma cell cytotoxic assay, inhibition of PTPN3 expression in mature DCs induced cytotoxic T lymphocytes with increased production of INF-γ and granzyme B, and enhanced toxicity against cancer cells and migration to cancer. Furthermore, inhibition of PTPN3 expression activated the mitogen-activated protein kinase pathway in DCs.
Conclusion
Based on our findings, inhibition of PTPN3 expression could contribute to the development of novel cancer immunotherapies that activate not only lymphocytes but also DCs.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PTPN:
-
Protein tyrosine phosphatase non-receptor type
- DC:
-
Dendritic cell
- siRNA:
-
Short interfering RNA
- IL:
-
Interleukin
- HLA:
-
Human leukocyte antigen
- IFN:
-
Interferon
- MAPK:
-
Mitogen-activated protein kinase
- PD-1:
-
Programmed cell death receptor-1
- PD-L1:
-
Programmed cell death ligand-1
- CTLA-4:
-
Cytotoxic T-lymphocyte associated antigen-4
- LAG3:
-
Lymphocyte activation gene 3
- irAE:
-
Immune-related adverse events
- APC:
-
Antigen-presenting cell
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- PBMC:
-
Peripheral blood mononuclear cell
- TNF:
-
Tumor necrosis factor
- CCL:
-
Chemokine ligand
- FACS:
-
Fluorescence‑activated cell sorting
- FITC:
-
Fluorescein isothiocyanate
- PE:
-
Phycoerythrin
- PCR:
-
Polymerase chain reaction
- CCR:
-
Chemokine receptor
- CTLs:
-
Cytotoxic T lymphocytes
- NK cell:
-
Natural killer cell
References
Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T (2004) Protein tyrosine phosphatases in the human genome. Cell 117(6):699–711. https://doi.org/10.1016/j.cell.2004.05.018
Amos SM et al (2011) Autoimmunity associated with immunotherapy of cancer. Blood 118(3):499–509. https://doi.org/10.1182/blood-2011-01-325266
Arimoto-Miyamoto K, Kadowaki N, Kitawaki T, Iwata S, Morimoto C, Uchiyama T (2010) Optimal stimulation for CD70 induction on human monocyte-derived dendritic cells and the importance of CD70 in naive CD4+ T-cell differentiation. Immunology 130(1):137–149. https://doi.org/10.1111/j.1365-2567.2010.03220.x
Borghaei H et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/nejmoa1507643
Chen TT (2013) Statistical issues and challenges in immuno-oncology. J Immunother Cancer. https://doi.org/10.1186/2051-1426-1-18
Chen YNP et al (2016) Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 535(7610):148–152. https://doi.org/10.1038/nature18621
Fujimura A et al (2019) PTPN3 expressed in activated T lymphocytes is a candidate for a non-antibody-type immune checkpoint inhibitor. Cancer Immunol Immunother 68(10):1649–1660. https://doi.org/10.1007/s00262-019-02403-y
Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM (2003) Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun 312(4):1236–1243. https://doi.org/10.1016/j.bbrc.2003.11.070
Huang J et al (2011) Irradiation enhances human T-cell function by upregulating CD70 expression on antigen-presenting cells in vitro. J Immunother 34(4):327–335. https://doi.org/10.1097/CJI.0b013e318216983d
Kiertscher SM, Gitlitz BJ, Figlin RA, Rothi MD (2003) Granulocyte/macrophage-colony stimulating factor and interleukin-4 expand and activate type-1 dendritic cells (DC1) when administered in vivo to cancer patients. Int J Cancer 107(2):256–261. https://doi.org/10.1002/ijc.11379
Lion E, Smits ELJM, Berneman ZN, Van Tendeloo VFI (2012) NK cells: key to success of DC-based cancer vaccines? Oncologist 17(10):1256–1270. https://doi.org/10.1634/theoncologist.2011-0122
López-Cotarelo P et al (2015) A novel MEK-ERK-AMPK signaling axis controls chemokine receptor CCR7-dependent survival in human mature dendritic cells. J Biol Chem 290(2):827–840. https://doi.org/10.1074/jbc.M114.596551
Ogino T, Onishi H, Suzuki H, Morisaki T, Tanaka M, Katano M (2012) Inclusive estimation of complex antigen presentation functions of monocyte-derived dendritic cells differentiated under normoxia and hypoxia conditions. Cancer Immunol Immunother 61(3):409–424. https://doi.org/10.1007/s00262-011-1112-5
Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14(12):1212–1218. https://doi.org/10.1038/ni.2762
Reck M et al (2021) (2021) First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 6(5):100273. https://doi.org/10.1016/j.esmoop.2021.100273
Riegel K et al (2020) RAF kinases are stabilized and required for dendritic cell differentiation and function. Cell Death Differ 27(4):1300–1315. https://doi.org/10.1038/s41418-019-0416-4
Rodríguez-Fernández JL, Criado-García O (2020) The chemokine receptor CCR7 uses distinct signaling modules with biased functionality to regulate dendritic cells. Front Immunol. https://doi.org/10.3389/fimmu.2020.00528
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179(4):1109–1118. https://doi.org/10.1084/jem.179.4.1109
Socinski MA et al (2018) Atezolizumab for First-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. https://doi.org/10.1056/nejmoa1716948
Steinman RM, Cohn ZA (1973) Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137(5):1142–1162. https://doi.org/10.1084/jem.137.5.1142
Steinman RM, Pope M (2002) Exploiting dendritic cells to improve vaccine efficacy. J Clin Investig 109(12):1519–1526. https://doi.org/10.1172/jci15962
Tawbi HA et al (2022) Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 386(1):24–34. https://doi.org/10.1056/nejmoa2109970
Trinchieri G (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3(2):133–146. https://doi.org/10.1038/nri1001
Zhao Y et al (2019) PD-L1:CD80 cis-heterodimer triggers the co-stimulatory receptor CD28 while repressing the inhibitory PD-1 and CTLA-4 pathways. Immunity 51(6):1059-1073.e9. https://doi.org/10.1016/j.immuni.2019.11.003
Acknowledgements
We would like to thank Ms. Emi Onishi for technical assistance. We also thank Editage for the English language review. This study was supported by the Japan Society for the Promotion of Science (KAKENHI Grant Number FAG21K08672).
Funding
This study was supported by the Japan Society for the Promotion of Science (KAKENHI Grant Number JP21K08672, JP21K09583, JP22H03163, JP22K08715).
Author information
Authors and Affiliations
Contributions
NI carried out the analysis of all experiments. SM, AI, KS, SM, SN, and SK were involved in the acquisition and analysis of data. HO, KO, MU, TM, and MN participated in the design of study. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All the procedures involving human participants performed in this study were in accordance with the ethical standards of the Kyushu University Ethics Committee (study approval numbers 29-251) and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All animal procedures were performed in accordance with the ethical standards of the Animal Care and Use Committee of Kyushu University (study approval number: A22-278-0).
Informed consent
Written informed consent was obtained from all the participants included in the study for the use of their PBMC and tumor specimen for research and for publication before blood collection.
Consent to participate
Written informed consent was obtained from all participants included in the study for the use of their PBMC and tumor specimen for research and for publication before blood collection.
Animal source
All mice were obtained from Charles River Laboratories Japan (Yokohama, Japan).
Cell line authentication
SBC5 cell line was obtained from the Japanese Collection of Research Bioresources bank. The Sq-1 cell line was purchased from Riken Bio Resource Research Center. All cell lines were cultured for no more than 2–3 weeks after thawing, routinely checked for mycoplasma infection, and showed consistent phenotypes by microscopy prior to the in vitro and in vivo experiments.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwamoto, N., Onishi, H., Masuda, S. et al. PTPN3 inhibition contributes to the activation of the dendritic cell function to be a promising new immunotherapy target. J Cancer Res Clin Oncol 149, 14619–14630 (2023). https://doi.org/10.1007/s00432-023-05250-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05250-8