Abstract
Purpose
Because patients younger than 40 years are rarely enrolled in clinical trials in non-small cell lung cancer (NSCLC), their survival benefit of immune checkpoint inhibitors (ICIs) needs to be clarified.
Methods
The National Cancer Database was queried for patients who were diagnosed with stage IV NSCLC between 2016 and 2018. ICIs were administered in the first-line setting. The overall survival (OS) of patients with stage IV NSCLC according to the receipt of ICIs was compared in different age groups (< 40, 40–49, 50–59, 60–69, 70–79, and ≥ 80 years). Multivariate analyses identified the clinical characteristics predictive of OS. Propensity score matching (PSM) was conducted to reduce the biases arising from clinical characteristics.
Results
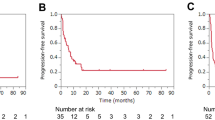
This study included 126,476 patients with stage IV NSCLC. In univariate analysis, ICI treatment was not associated with a survival benefit in patients younger than 40 years with stage IV NSCLC relative to their ICI-naïve counterparts after PSM (median OS: 24.2 months vs. 24.0 months, hazard ratio [HR] = 1.01, 95% confidence interval [CI] = 0.81–1.27, P = 0.9031). Multivariate analysis revealed that ICI use was not an independent predictor of OS in patients with stage IV NSCLC < 40 years old (HR = 0.96, 95% CI = 0.76–1.21, P = 0.7230). Sequential improvement of the HR was observed with increasing age.
Conclusion
Our study suggested a poor survival benefit of ICIs in stage IV NSCLC patients younger than 40 years old, which should be validated in prospective studies.




Similar content being viewed by others
Data availability
Data can be available upon request to NCDB.
References
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Özgüroğlu M (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379(24):2342–2350
Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K (2018) Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 19(11):1468–1479
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, Ready N, Hiltermann TJN, Nair S, Juergens R, Peters S, Minenza E, Wrangle JM, Rodriguez-Abreu D, Borghaei H, Blumenschein GR Jr, Villaruz LC, Havel L, Krejci J, Corral Jaime J, Chang H, Geese WJ, Bhagavatheeswaran P, Chen AC, Socinski MA (2017a) First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376(25):2415–2426
Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S (2019) Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 30(1):44–56
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387(10030):1837–1846
Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, Han JY, Gadgeel SM, Hida T, Cortinovis DL, Cobo M, Kowalski DM, De Marinis F, Gandhi M, Danner B, Matheny C, Kowanetz M, He P, Felizzi F, Patel H, Sandler A, Ballinger M, Barlesi F (2018) Updated efficacy analysis including secondary population results for OAK: a randomized phase III Study of atezolizumab versus docetaxel in patients with previously treated advanced non-small cell lung cancer. J Thorac Oncol 13(8):1156–1170
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro Jr G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550
Komiya T, Powell E, Takamori S (2021a) Prognostic impact of single and multiple descriptors in pathologically staged T3N0M0 NSCLC. JTO Clin Res Rep 2(3):100111
Komiya T, Powell E, Takamori S (2021b) Role of thoracic radiation in extensive stage small cell lung cancer: a NCDB analysis. Med Oncol 38(4):44
Komiya T, Takamori S, Wilding G (2022) Adjuvant radiation therapy improves survival in stage IIIA (N2) non-small cell lung cancer with persistent N2 disease after neoadjuvant chemotherapy. Radiother Oncol 176:234–238
Kugel CH 3rd, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, Weiss SA, Darvishian F, Al-Rohil RN, Ndoye A, Behera R, Alicea GM, Ecker BL, Fane M, Allegrezza MJ, Svoronos N, Kumar V, Wang DY, Somasundaram R, Hu-Lieskovan S, Ozgun A, Herlyn M, Conejo-Garcia JR, Gabrilovich D, Stone EL, Nowicki TS, Sosman J, Rai R, Carlino MS, Long GV, Marais R, Ribas A, Eroglu Z, Davies MA, Schilling B, Schadendorf D, Xu W, Amaravadi RK, Menzies AM, McQuade JL, Johnson DB, Osman I, Weeraratna AT (2018) Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res 24(21):5347–5356
Li P, Che S, Qi Y, Luo N, Lin Q, Zhu X, Xuan Y, Li M, Li J, Ge M, Sun T, Qi C, Wang Y (2022) Age-dependent genomic characteristics and their impact on immunotherapy in lung adenocarcinoma. J Cancer Res Clin Oncol 149:2997–3007
Liu B, Quan X, Xu C, Lv J, Li C, Dong L, Liu M (2019) Lung cancer in young adults aged 35 years or younger: a full-scale analysis and review. J Cancer 10(15):3553–3559
Marur S, Singh H, Mishra-Kalyani P, Larkins E, Keegan P, Sridhara R, Blumenthal GM, Pazdur R (2018) FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Semin Oncol 45(4):220–225
McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, Jonasch E, Khasraw M, Heimberger AB, Lim B, Ueno NT, Litton JK, Ferrarotto R, Chang JT, Moulder SL, Lin SY (2021) High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol 32(5):661–672
Mogilenko DA, Shpynov O, Andhey PS, Arthur L, Swain A, Esaulova E, Brioschi S, Shchukina I, Kerndl M, Bambouskova M, Yao Z, Laha A, Zaitsev K, Burdess S, Gillfilan S, Stewart SA, Colonna M, Artyomov MN (2021) Comprehensive profiling of an aging immune system reveals clonal GZMK(+) CD8(+) T cells as conserved hallmark of inflammaging. Immunity 54(1):99-115.e12
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393(10183):1819–1830
Nikolich-Žugich J (2018) The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 19(1):10–19
Nosaki K, Saka H, Hosomi Y, Baas P, de Castro Jr G, Reck M, Wu YL, Brahmer JR, Felip E, Sawada T, Noguchi K, Han SR, Piperdi B, Kush DA, Lopes G (2019) Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 135:188–195
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A, Sakai H, Lingua A, Salman P, Souquet PJ, De Marchi P, Martin C, Pérol M, Scherpereel A, Lu S, John T, Carbone DP, Meadows-Shropshire S, Agrawal S, Oukessou A, Yan J, Reck M (2021) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 22(2):198–211
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Vandormael K, Riccio A, Yang J, Pietanza MC, Brahmer JR (2019) Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol 37(7):537–546
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389(10066):255–265
Sacher AG, Dahlberg SE, Heng J, Mach S, Jänne PA, Oxnard GR (2016) Association between younger age and targetable genomic alterations and prognosis in non–small-cell lung cancer. JAMA Oncol 2(3):313–320
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301
Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T (2012) Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 24(5):331–341
Takamori S, Komiya T, Powell E (2021a) Survival benefit from immunocheckpoint inhibitors in stage IV non-small cell lung cancer patients with brain metastases: a National Cancer Database propensity-matched analysis. Cancer Med 10(3):923–932
Takamori S, Takada K, Shimokawa M, Matsubara T, Fujishita T, Ito K, Toyozawa R, Yamaguchi M, Okamoto T, Yoneshima Y, Tanaka K, Okamoto I, Tagawa T, Mori M (2021b) Clinical utility of pretreatment Glasgow prognostic score in non-small-cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer 152:27–33
Takamori S, Komiya T, Powell E (2022) Clinical impact of number of lymph nodes dissected on postoperative survival in node-negative small cell lung cancer. Front Oncol 12:962282
Takamori S, Komiya T, Shimokawa M, Powell E (2023) Lymph node dissections and survival in sublobar resection of non-small cell lung cancer ≤ 20 mm. Gen Thorac Cardiovasc Surg 71(3):189–197
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20(7):924–937
Wu Q, Wang Q, Tang X, Xu R, Zhang L, Chen X, Xue Q, Wang Z, Shi R, Wang F, Ju F, Zhang B, Zhou YL (2019) Correlation between patients’ age and cancer immunotherapy efficacy. Oncoimmunology 8(4):e1568810
Yamaguchi O, Imai H, Minemura H, Suzuki K, Wasamoto S, Umeda Y, Osaki T, Kasahara N, Uchino J, Sugiyama T, Ishihara S, Ishii H, Naruse I, Mori K, Kotake M, Kanazawa K, Minato K, Kagamu H, Kaira K (2020) Efficacy and safety of immune checkpoint inhibitor monotherapy in pretreated elderly patients with non-small cell lung cancer. Cancer Chemother Pharmacol 85(4):761–771
Zappa C, Mousa SA (2016) Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 5(3):288–300
Acknowledgements
We thank Joe Barber Jr., PhD, from Edanz (https://jp.edanz.com/ac) and Gregory Wilding for editing a draft of this manuscript and technical assistance, respectively.
Funding
There are no sources of funding to report.
Author information
Authors and Affiliations
Contributions
ST wrote the mαin manuscript text, TK. performed statistical analysis, and MS supervised all the statistical analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Takefumi Komiya received institutional research funding from Merck and Gilead and advisory fees from Regeneron and Novocure. All authors declare no conflicts of interest associated with this study.
Ethical approval
Based on the use of only de-identified data, the study was exempted by the Parkview institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2023_5167_MOESM1_ESM.pptx
Supplementary Fig. 1. The Kaplan–Meier curve of overall survival among patients with stage IV non-small cell lung cancer according to immune checkpoint inhibitor use. Supplementary file1 (PPTX 39 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takamori, S., Shimokawa, M. & Komiya, T. Efficacy of immune checkpoint inhibitors in younger patients with non-small cell lung cancer. J Cancer Res Clin Oncol 149, 13175–13184 (2023). https://doi.org/10.1007/s00432-023-05167-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05167-2