Abstract
Objectives
The COVID-19 pandemic affected medical care for chronic diseases. This study aimed to systematically assess the pandemic impact on oncological care in Germany using a rapid review.
Methods
MEDLINE, Embase, study and preprint registries and study bibliographies were searched for studies published between 2020 and 2 November 2022. Inclusion was based on the PCC framework: population (cancer), concept (oncological care) and context (COVID-19 pandemic in Germany). Studies were selected after title/abstract and full-text screening by two authors. Extracted data were synthesized using descriptive statistics or narratively. Risk of bias was assessed and summarized using descriptive statistics.
Results
Overall, 77 records (59 peer-reviewed studies and 18 reports) with administrative, cancer registry and survey data were included. Disruptions in oncological care were reported and varied according to pandemic-related factors (e.g., pandemic stage) and other (non-pandemic) factors (e.g., care details). During higher restriction periods fewer consultations and non-urgent surgeries, and delayed diagnosis and screening were consistently reported. Heterogeneous results were reported for treatment types other than surgery (e.g., psychosocial care) and aftercare, while ongoing care remained mostly unchanged. The risk of bias was on average moderate.
Conclusions
Disruptions in oncological care were reported during the COVID-19 pandemic in Germany. Such disruptions probably depended on factors that were insufficiently controlled for in statistical analyses and evidence quality was on average only moderate. Research focus on patient outcomes (e.g., longer term consequences of disruptions) and pandemic management by healthcare systems is potentially relevant for future pandemics or health emergencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic contributed to rapid reorganization of medical care for chronic diseases, such as cancer in Germany and elsewhere (Scheidt-Nave et al. 2021). Emergency care for COVID-19 cases was prioritized, while other hospital admissions, planned medical treatments or existing care for chronic diseases were adapted to the pandemic conditions, postponed or cancelled. Disruptions in oncological care were reported in Europe, North America and other world regions (The Lancet Oncology 2020). Missed or delayed cancer screening or treatment could lead to an increase in symptomatic cancer patients (Jones et al. 2020) or worse outcomes (Raphael et al. 2016) as well as more cancer-related deaths (Joung et al. 2022).
Conflicting results regarding oncological care were reported since the onset of the COVID-19 pandemic in Germany. Perceived disruptions in medical care for chronically ill or people of advanced age (Heidemann et al. 2020) and disruptions in oncological care were reported (Weisel et al. 2020). Specifically, a scoping review covering the first few months of the COVID-19 pandemic reported a reduced number of consultations, inpatient admissions and tumor diagnostic procedures as well as fewer primary surgeries for some cancer entities in Germany (Scheidt-Nave et al. 2021). However, these disruptions were not perceived as critical and probably affected only a small number of time-critical therapies at the time (Scheidt-Nave et al. 2021). Nearly three years into the pandemic (i.e. in October 2022) a coherent picture of the characteristics and extent of disruptions in oncological care provision is still missing in Germany, partly due to the heterogeneity in data sources and reported data, but also because a comprehensive assessment of disruptions is possible only after sufficient time has passed. Thus, this study aimed to systematically assess the impact of the COVID-19 pandemic on oncological care in Germany using rapid review methods.
Materials and methods
Review design and protocol
This study is a rapid review based on the methodological recommendations from Cochrane (Garritty et al. 2021). Rapid review methodology is appropriate to address urgent health issues with high priority (Garritty et al. 2021). Since our aim was to map the existing literature by broadly focusing on any aspect of oncological care (e.g., data sources, sample and cancer types, periods of data collection and clinical settings), we follow the recommendations for scoping reviews to chart and synthesize the data (Pollock et al. 2022; Tricco et al. 2018). The reporting adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) checklist (Tricco et al. 2018); Supplementary Information, Table S1. A protocol for this review was prospectively registered at the Open Science Framework (osf.io/f2dnj). There were no changes between the protocol and this review.
Eligibility criteria
Inclusion was based on the PCC framework: (1) population: cancer patients (any age), people eligible for screening or healthcare professionals in oncology, (2) concept: oncological care (delivery and utilization), (3) context: the COVID-19 pandemic in Germany (i.e., March 2020 through October 2022 with or without pre-pandemic comparison period), (4) study type: primary studies published in peer-reviewed journals with any study design and data type, reports without peer-review (e.g., health insurance claims data) or preprints; in English or German language and available in full-text. Conference papers, dissertations, reviews, and commentaries were excluded.
Information sources
The information sources were bibliographic databases (MEDLINE and Embase), bibliographies of included studies, online registers of COVID-19 studies or preprints, and reports without peer-review (via relevant organization websites). Syntax and search strategies are reported in Supplementary Information, Tables S2-S4.
Search strategy
The search syntax was developed and calibrated within the team. MEDLINE and Embase were searched on 2 November 2022 by experienced librarians while the other information sources were searched through 15 November 2022 by the authors. Search results were stored and managed in EndNote X9 and Covidence.
Selection of sources of evidence
Title/abstract and full-text screening were performed independently by two authors and relevant studies were selected by consensus.
Data charting
Peer-reviewed studies were coded by one author and checked by another author using a self-developed data charting form in Excel 10. The data were coded into pre-defined categories or charted into themes that inductively emerged from studies according to recommendations for scoping reviews (Pollock et al. 2022). Reports without peer-review were narratively coded by one author.
Data items
Data items included bibliographic information, study characteristics (study design, data source, region in Germany, data collection period), sample characteristics (sample type, cancer type), oncological care (care aspects, study outcomes, factors associated with care) and evidence gaps. Based on descriptive patterns in study outcomes, oncological care was classified as either ‘no changes in care’ or ‘disruptions in care’.
Critical appraisal (risk of bias assessment)
Based on recommendations for rapid reviews (Garritty et al. 2021), we performed a risk of bias assessment to evaluate the evidence quality in peer-reviewed studies using validated instruments from Cochrane and JBI or additional bespoke instruments for specific study types (e.g., modelling studies; Supplementary Information, Table S5). Each study was independently assessed by two authors and any disagreements were resolved by consensus. In general, items indicating a low risk of bias (i.e., fulfilled or not applicable items) were scored as 1 and items indicating a high risk of bias (i.e., not fulfilled, not reported or unclearly reported items) were scored as 0. For example, an item was fulfilled and scored as 1 if a study controlled for confounders in a statistical analysis. A mean risk of bias score was computed for all studies as the sum of all items rated 1 out of all items divided by the number of studies.
Data synthesis
We synthesized the data using descriptive statistics or narrative descriptions of common themes.
Stakeholder involvement
We discussed the data items with a relevant stakeholder (a counselor at the Bremen Cancer Society) to ensure that we address the most important aspects of oncological care in this rapid review.
Results
Study selection
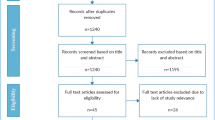
From 6196 records identified via database searches and 788 records identified via other methods, 77 records met the inclusion criteria (Fig. 1). These included 59 peer-reviewed studies (Arndt et al. 2022; Balakirski et al. 2022; Balk et al. 2022; Bartella et al. 2021; Beller et al. 2022; Bollmann et al. 2021; Brunner et al. 2020; Buntzel et al. 2020, 2021; Dienemann et al. 2021; Diers et al. 2021, 2022; Donath et al. 2021; Eckford et al. 2021; Erdmann et al. 2021, 2022; Fauser et al. 2022; Gremke et al. 2022; Griewing et al. 2022a, b; Gschnell et al. 2021; Haier et al. 2022a, b, c; Hajek et al. 2021; Harke et al. 2020, 2022; Heimes et al. 2021; Holzel et al. 2022; Hunger et al. 2022; Jacob et al. 2021, 2022; Jördens et al. 2021; Justenhoven and Rieger 2022; Kaltofen et al. 2022; Kapsner et al. 2020; Kirchberg et al. 2021; Kleemann et al. 2022; Kourtidis et al. 2022; Kuhlen et al. 2020; Matuschek et al. 2020; Medenwald et al. 2022; Micek et al. 2022; Michalowsky et al. 2021; Piontek et al. 2021; Reichardt et al. 2021; Riemann et al. 2021; Rupa et al. 2020; Schuz et al. 2022; Stang et al. 2020; Stos et al. 2020; Struck et al. 2022; Teuscher et al. 2022; Voigtlander et al. 2021; Vu et al. 2022; Walter et al. 2021, 2022; Wang et al. 2020; Ziegler et al. 2022) and 18 reports without peer-review (Acar et al. 2021; Deutsche Krebsgesellschaft Deutsche Krebshilfe Deutsches Krebsforschungszentrum 2021a, b; Fröhling and Arndt 2020; Günster et al. 2020; Heidt et al. 2021; Hermes-Moll et al. 2021; Klinische Krebsregister Sachsen 2022; Klinisches Krebsregister für Brandenburg und Berlin 2022a, b; Mangiapane et al. 2021, 2022; Mostert et al. 2021; Rückher and Pflüger 2022; Tillmanns et al. 2022; Wissenschaftliches Institut der AOK WIdO 2021, 2022; Zok 2021). A list of excluded studies is reported in Supplementary Information, Table S6. All data are reported in Supplementary Information, Tables S7-S8.
Bibliographic characteristics
The 59 peer-reviewed studies were published between 2020 and 2022. The studies reported either no conflicts of interest due to funding (43/59) or did not report the sources of funding (16/59).
Study characteristics
All 59 peer-reviewed studies were based on administrative, cancer registry or survey data in Germany (Fig. 2). Most studies included patients of any age and with any cancer type (already diagnosed or in the screening or detection stage).
Oncological care during the COVID-19 pandemic
Oncological care was assessed either solely over the course of the COVID-19 pandemic (i.e., March 2020 onwards) in all studies or in relation to different pre-pandemic periods (i.e., before March 2020) in 39/59 studies. Oncological care was delivered in any clinical setting based on location (e.g., general hospital, specialized clinic, medical practice, rehabilitation facility) and provision (i.e., in- or outpatient care). Five care aspects inductively emerged from the studies:
-
1.
Any (general or unspecified) care: consultations, appointments, hospitalizations, and related details (e.g., length of hospital stay, restrictions in care, mental burden)
-
2.
Diagnosis: screening, incidence or detection of tumor or metastases
-
3.
Treatment: surgery, radiotherapy, systemic therapy or psychosocial care
-
4.
Aftercare: follow-up treatment or rehabilitation
-
5.
Other (specific) care: palliative care and related outcomes (survival rate or mortality)
Since this review is based on data from one country and one disease group (i.e., cancer) there is a risk that the same patients or healthcare professionals were included in multiple studies. To reduce double counting, we clustered the studies that used the same or similar data sources (e.g., same hospital groups or databases) or the same regions in Germany into groups. We then assessed and reported oncological care within each group of studies.
Based on 32 studies with administrative data (cited in Supplementary Information, Table S9) and eight studies with cancer registry data (cited in Supplementary Information, Table S10) collected nationwide or regionally, oncological care was temporarily disrupted throughout 2020–2021, although some studies did not report any changes relative to 2019 or earlier (Table 1). Disruptions were reported during periods of high restrictions and some recovered after restrictions were relaxed. Most disruptions were detected for low-risk patients (e.g., delays in screening and non-urgent surgery) while ongoing treatment was unchanged. Most consistent disruptions were reported for any general or unspecified care (e.g., patient volume), diagnosis (e.g., detection or screening) and treatment (e.g., surgery). Treatment types other than surgery (e.g., psychosocial care) and aftercare (e.g., follow-up treatment) were less often assessed and there were mixed results. Any disruptions in oncological care depended on pandemic stage (i.e., periods of high or low restrictions), institution type (e.g., hospitals or outpatient facilities), region in Germany, cancer type and stage, and patient characteristics. Furthermore, 21 studies with survey data (cited in Supplementary Information, Table S11) confirmed the trends seen in studies with administrative and registry data (Table 1). Patients and healthcare professionals reported fewer consultations and disruptions in appointments (e.g., delays in screening), reduced access to clinical facilities for patients and accompanying persons and higher mental burden related to treatment uncertainties. Healthcare professionals reported higher workload, mental burden and disruptions in clinical management (e.g., changes in clinical processes, limited resources and communication with patients).
In addition to peer-reviewed studies, we also included 18 reports without peer-review (cited in Supplementary Information, Table S12) based on claims, survey, cancer registry data, or unsolicited feedback. These resources were published as reports, magazine articles, or press releases. Reports with hospital data mentioned disruptions in admissions, therapeutic colonoscopies and tumor surgeries with every wave of SARS-CoV-2 infections. Such disruptions varied by pandemic phase, treatment setting (inpatient or outpatient) and procedure. The inpatient setting was more heavily affected than the outpatient setting. Reports discussed a shift in resource allocation within hospitals and between patient groups. In contrast, reports with claims data from the outpatient practice sector showed that case numbers remained stable or tended to increase. Reports with cancer registry data were mostly preliminary. The number of cancer screening examinations varied by cancer type and was affected by temporary suspensions and adaptations of screening programs. Reports with survey data indicate that screening-eligible individuals experienced cancellation or postponement of screening appointments by their healthcare providers. Healthcare providers stated that while the pandemic negatively affected some care aspects, such as follow-up and psychosocial care, acute cancer care was maintained at pre-pandemic levels.
Risk of bias
The risk of bias was on average moderate (Table 1; Supplementary Information, Tables S13-S16). The most important source of the high risk of bias was that confounding factors were inadequately controlled for when assessing or interpreting the changes in oncological care during the COVID-19 pandemic.
Factors potentially associated with disruptions in oncological care
Studies included in this review suggest that any disruptions in oncological care in Germany during the COVID-19 pandemic were associated with the pandemic itself (i.e., pandemic-related factors) and other (non-pandemic) factors (Table 2). Some of these factors were controlled for in statistical analyses, while others were mentioned by study authors in discussion, limitations or conclusions.
Evidence gaps and topics for future research
Based on the included studies, we inductively identified evidence gaps and topics for future research that focus on patient health outcomes and pandemic management by healthcare systems (Table 3).
Discussion
Overall summary
Consistent with global disruptions in oncological care (The Lancet Oncology 2020), such disruptions in oncological care were also reported in Germany according to 77 records (59 peer-reviewed studies and 18 reports) included in this rapid review. The disruptions varied according to pandemic-related factors (e.g., pandemic stage) and other (non-pandemic) factors (e.g., care details). During higher restriction periods fewer consultations and non-urgent surgeries, and delayed diagnosis and screening were consistently reported. Heterogeneous results were reported for treatment types other than surgery (e.g., psychosocial care) and aftercare, while ongoing care remained mostly unchanged. The risk of bias was on average moderate.
Extent of disruptions in oncological care during the COVID-19 pandemic
As suggested by others (Dienemann et al. 2021) and based on studies in this review, disruptions in oncological care reported during the COVID-19 pandemic in Germany probably depended on various pandemic-related and other factors, such as patient and disease characteristics, as well as setting and care details. For example, reduced patient volume especially during the high restriction periods might have been due to pandemic-related reduction in utilization of care (Scheidt-Nave et al. 2021) or to pandemic-unrelated reorganization of care within healthcare institutions (Dinkel et al. 2021; Weisel et al. 2020). In Germany, patient volume at specialist clinics could be affected by difficulties in access to care upstream from such clinics. This is because in Germany patients typically obtain a referral for a specialist consultation from their general practitioners. Thus, reduced access to general practitioners during the pandemic might have contributed to fewer referrals to specialist care and thus lower patient volume. In general, it is difficult to establish to what extent the COVID-19 pandemic affected the oncological care because most studies in this review used data from different pandemic periods and lacked detailed data on other factors.
Future research
Studies included in this review suggest that patient outcomes related to disruptions in oncological care should be investigated in future research. The ethics of prioritization in medicine and resource allocation that contributed to disruptions in care for chronic diseases have already been questioned (Brunner et al. 2020; Eckford et al. 2021). Delays in cancer detection and treatment are associated with detrimental health effects (Alkatout et al. 2021; Hanna et al. 2020) and are predicted to contribute to higher mortality (Maringe et al. 2020). Furthermore, the patient perspective with respect to psychosocial aspects and care expectations during health emergencies needs to be considered in future research (Dinkel et al. 2021). As shown in this review and other studies (Bauerle et al. 2021; Colomer-Lahiguera et al. 2021; Verma et al. 2022; Ziegler et al. 2022), cancer patients reported a high mental burden of the COVID-19 pandemic due to uncertainties regarding their treatment and restrictions in public life (e.g., visiting restrictions, loneliness and reduction in psychosocial support).
Future studies should also evaluate the effectiveness of pandemic management measures in preparation for any future health emergencies (Weisel et al. 2020). Adaptation of organizational processes was identified as an important measure to maintain the usual oncological care provision even in regions with high incidence of SARS-CoV-2 infections in the early stages of the pandemic in Germany (Akuamoa-Boateng et al. 2020). Various measures to prevent SARS-CoV-2 infections were also necessary to reduce potentially detrimental effects of such infections in people with cancer (Tang and Hu 2020). As sufficient data become available, both health consequences and economic implications of such measures need to be evaluated (Goldsbury et al. 2018; von Dercks et al. 2020). Furthermore, as shown in this review and discussed by others (Beller et al. 2022), pandemic management measures should also focus on healthcare providers with the aim to reduce the workload and the mental burden in oncological health professionals that were reported during the COVID-19 pandemic in Germany.
Strengths and limitations
The main strength of this review is a large volume of the included literature (59 peer-reviewed studies and 18 reports) based on nationwide data from a single country (Germany) with a highly-developed healthcare system. The results of this review show how this healthcare system managed the oncological care under the strain of a worldwide COVID-19 pandemic. Since the COVID-19 pandemic seems to have ended in most parts of the world, the review deals with a historical health event. Thus, the results of this review are potentially relevant for management of any future pandemics and health emergencies. Furthermore, we identified several factors that could be considered when evaluating longitudinal data on the impact of health emergencies in one clinical field on care patterns for chronic diseases in other fields.
There were several limitations in this review. First, study results were difficult to synthesize due to heterogeneous outcomes and data collection periods during which different pandemic-related restrictions were imposed in Germany (Supplementary Information, Table S17). Second, the evidence quality in this review was on average only moderate based on the risk of bias assessment. Factors that might have affected oncological care patterns over time (e.g., changes in screening programs) were inadequately controlled for in descriptive statistical analyses of longitudinal data collected during the COVID-19 pandemic. While administrative and registry data were affected by delays in data entry and lacked detailed clinical and sociodemographic patient characteristics, survey data may have overestimated the disruptions in care (e.g., pediatricians estimated about 40% fewer consultations, while hospital data showed 30% fewer consultations in one survey (Donath et al. 2021)). Third, as a consequence of the first two limitations, this review qualitatively describes any trends in data because we could not estimate the standardized effect sizes and variance. Computation of effect sizes was not possible because absolute rather than relative data were reported (e.g., absolute patient volume without total patient volume admitted to a clinical facility) and comparison time periods before the pandemic were heterogeneous or not included in the study. Thus, it is unclear if small absolute changes in oncological care are clinically meaningful or to what extent they deviate from the natural fluctuation observed in medical care for chronic diseases. A planned meta-analysis was not performed due to heterogeneous samples, data collection and comparison time periods, and outcomes of oncological care. Fourth, it cannot be ruled out that the same patients were included in multiple studies that used nationwide data, similar data collection periods and overlapping cancer and care types. However, such overlap probably had little consequences on our results because we described the outcomes qualitatively. Fifth, this highly sensitive and politicized field of prioritization in medicine could have been affected by publication bias toward studies reporting disruptions in oncological care. While adaptation of organizational processes contributed to effective oncological care provision during the early stages of the pandemic (Akuamoa-Boateng et al. 2020), other studies not reporting any disruptions may not have been published. Thus, the generalizability of the results of this review beyond the 77 included records is unclear.
Conclusions
Disruptions in oncological care were reported during the COVID-19 pandemic in Germany according to 77 records. Such disruptions depended on factors that were insufficiently controlled for and evidence quality was on average only moderate. Research focus on patient outcomes (e.g., longer term consequences of disruptions) and pandemic management by healthcare systems is potentially relevant for future pandemics or health emergencies.
Data availability
All data reported in this review are shown in the online Supplementary Information.
References
Acar L, L‘hoest H, Marschall U (2021) Der Einfluss der Coronapandemie auf die medizinische Versorgung schwerwiegender Erkrankungen im Jahr 2020 (report). Gesundheitswesen aktuell, BARMER Institut für Gesundheitssystemforschung (bifg). https://doi.org/10.30433/GWA2021-308. Accessed 26 Oct 2022
Akuamoa-Boateng D, Wegen S, Ferdinandus J, Marksteder R, Baues C, Marnitz S (2020) Managing patient flows in radiation oncology during the COVID-19 pandemic: reworking existing treatment designs to prevent infections at a German hot spot area University Hospital. Strahlenther Onkol 196:1080–1085. https://doi.org/10.1007/s00066-020-01698-6
Alkatout I, Biebl M, Momenimovahed Z, Giovannucci E, Hadavandsiri F, Salehiniya H et al (2021) Has COVID-19 affected cancer screening programs? A Syst Rev. Front Oncol. 11:675038. https://doi.org/10.3389/fonc.2021.675038
Arndt V, Doege D, Frohling S, Albers P, Algul H, Bargou R et al (2022) Cancer care in German centers of excellence during the first 2 years of the COVID-19 pandemic. J Cancer Res Clin Oncol 14:14. https://doi.org/10.1007/s00432-022-04407-1
Balakirski G, Michalowitz AL, Kreuter A, Hofmann SC (2022) Long-term effects of the COVID-19 pandemic on malignant melanoma: increased lymph node metastases in two German dermatology clinics. J Eur Acad Dermatol Venereol 36:e762–e764. https://doi.org/10.1111/jdv.18337
Balk M, Rupp R, Craveiro AV, Allner M, Grundtner P, Eckstein M et al (2022) The COVID-19 pandemic and its consequences for the diagnosis and therapy of head and neck malignancies. Eur Rev Med Pharmacol Sci 26:284–290. https://doi.org/10.26355/eurrev_202201_27779
Bartella AK, Halama D, Kamal M, Hahnel S, Sander AK, Pausch NC et al (2021) Impact of COVID-19 on oral and maxillofacial surgery: preliminary results after the curfew. J Craniofac Surg 32:e305–e308. https://doi.org/10.1097/scs.0000000000007062
Bauerle A, Musche V, Schmidt K, Schweda A, Fink M, Weismuller B et al (2021) Mental health burden of german cancer patients before and after the outbreak of covid-19: predictors of mental health impairment. Int J Environ Res Public Health 18:26. https://doi.org/10.3390/ijerph18052318
Beller J, Schafers J, Geyer S, Haier J, Epping J (2022) Patterns of changes in oncological care due to covid-19: results of a survey of oncological nurses and physicians from the region of hanover. Germany Healthcare 10:22. https://doi.org/10.3390/healthcare10010015
Bollmann A, Hohenstein S, Pellissier V, Stengler K, Reichardt P, Ritz JP et al (2021) Utilization of in- and outpatient hospital care in Germany during the Covid-19 pandemic insights from the German-wide Helios hospital network. PLoS ONE 16:e0249251. https://doi.org/10.1371/journal.pone.0249251
Brunner M, Krautz C, Kersting S, Weber GF, Stinner B, Benz SR et al (2020) Oncological colorectal surgery during the COVID-19 pandemic- a national survey. Int J Colorectal Dis 35:2219–2225. https://doi.org/10.1007/s00384-020-03697-6
Buntzel J, Klein M, Keinki C, Walter S, Buntzel J, Hubner J (2020) Oncology services in corona times: a flash interview among German cancer patients and their physicians. J Cancer Res Clin Oncol 146:2713–2715. https://doi.org/10.1007/s00432-020-03249-z
Buntzel J, Micke O, Klein M, Buntzel J, Walter S, Keinki C et al (2021) Take care or “German Angst”? Lessons from cancer care during COVID-19 pandemic in spring 2020. J Cancer Res Clin Oncol 147:2093–2105. https://doi.org/10.1007/s00432-020-03492-4
Colomer-Lahiguera S, Ribi K, Dunnack HJ, Cooley ME, Hammer MJ, Miaskowski C et al (2021) Experiences of people affected by cancer during the outbreak of the COVID-19 pandemic: an exploratory qualitative analysis of public online forums. Support Care Cancer 29:4979–4985. https://doi.org/10.1007/s00520-021-06041-y
Deutsche Krebsgesellschaft Deutsche Krebshilfe Deutsches Krebsforschungszentrum (2021a) Universitätskliniken fürchten Triage bei Krebspatient*innen (press release). https://www.krebsgesellschaft.de/deutsche-krebsgesellschaft-wtrl/pressemitteilungen-2021a.triage.html. Accessed 26 Oct 2022
Deutsche Krebsgesellschaft Deutsche Krebshilfe Deutsches Krebsforschungszentrum (2021b) Versorgung von Krebspatient*innen hochgefährdet (press release). https://www.krebsgesellschaft.de/deutsche-krebsgesellschaft-wtrl/willkommen/presse/pressemitteilungen-2021b/versorgung-von-krebspatienten-hochgefaehrdet.html. Accessed 26 Oct 2022
Dienemann T, Brennfleck F, Dejaco A, Grutzmann R, Binder J, Krautz C et al (2021) Collateral effects of the SARS-CoV-2 pandemic on oncologic surgery in Bavaria. BMC Surg 21:411. https://doi.org/10.1186/s12893-021-01404-y
Diers J, Acar L, Baum P, Flemming S, Kastner C, Germer CT et al (2021) Fewer operations for cancer in Germany during the first wave of COVID-19 in 2020-A cohort study and time-series analysis. Dtsch Arztebl Int 118:481–482. https://doi.org/10.3238/arztebl.m2021.0265
Diers J, Acar L, Wagner JC, Baum P, Hankir M, Flemming S et al (2022) Cancer diagnosis is one quarter lower than the expected cancer incidence in the first year of COVID-19 pandemic in Germany: a retrospective register-based cohort study. Cancer Commun 42:673–676. https://doi.org/10.1002/cac2.12314
Dinkel A, Goerling U, Honig K, Karger A, Maatouk I, Petermann-Meyer A et al (2021) Psychooncological care for patients with cancer during 12 months of the Covid-19 pandemic: Views and experiences of senior psychooncologists at German comprehensive cancer centers. Psychooncology 30:1982–1985. https://doi.org/10.1002/pon.5759
Donath H, Zielen S, Wittekindt B, Klingebiel T, Graf J, Eckrich M et al (2021) Effects of the SARS-CoV2-Lockdown on pediatric care in the rhine-main area. Klin Padiatr 233:31–36. https://doi.org/10.1055/a-1263-1467
Eckford RD, Gaisser A, Arndt V, Baumann M, Kludt E, Mehlis K et al (2021) The COVID-19 pandemic and cancer patients in germany: impact on treatment, follow-up care and psychological burden. Front Public Health 9:788598. https://doi.org/10.3389/fpubh.2021.788598
Erdmann F, Wellbrock M, Trubenbach C, Spix C, Schrappe M, Schuz J et al (2021) Impact of the COVID-19 pandemic on incidence, time of diagnosis and delivery of healthcare among paediatric oncology patients in Germany in 2020: Evidence from the German Childhood Cancer Registry and a qualitative survey. Lancet Reg. 9:100188. https://doi.org/10.1016/j.lanepe.2021.100188
Erdmann F, Spix C, Schrappe M, Borkhardt A, Schuz J (2022) Temporal changes of the incidence of childhood cancer in Germany during the COVID-19 pandemic: updated analyses from the german childhood cancer registry. Lancet Reg. 17:100398. https://doi.org/10.1016/j.lanepe.2022.100398
Fauser D, Banaschak H, Zollmann P, Streibelt M, Bethge M (2022) Impact of the SARS-CoV-2 Pandemic on the utilization of cancer rehabilitation: a difference-in-differences analysis. Rehabilitation 24:24. https://doi.org/10.1055/a-1936-4083
Fröhling S, Arndt V (2020) Versorgung von Krebspatienten: Corona-Effekt in der Onkologie (magazine article). Dtsch Arztebl 117:A2234–42. https://www.aerzteblatt.de/archiv/216717/Versorgung-von-Krebspatienten-Corona-Effekt-in-der-Onkologie. Accessed 26 Oct 2022
Garritty C, Gartlehner G, Nussbaumer-Streit B, King VJ, Hamel C, Kamel C et al (2021) Cochrane rapid reviews methods group offers evidence-informed guidance to conduct rapid reviews. J Clin Epidemiol 130:13–22. https://doi.org/10.1016/j.jclinepi.2020.10.007
Goldsbury DE, Yap S, Weber MF, Veerman L, Rankin N, Banks E et al (2018) Health services costs for cancer care in Australia: Estimates from the 45 and Up Study. PLoS ONE 13:e0201552. https://doi.org/10.1371/journal.pone.0201552
Gremke N, Griewing S, Felgentreff M, Kostev K, Kalder M (2022) Impact of the Coronavirus Disease 2019 (COVID-19) Pandemic on Cervical Cancer Screening in Gynecological Practices in Germany. Cancers. https://doi.org/10.3390/cancers14194820
Griewing S, Wagner U, Lingenfelder M, Fischer R, Kalder M (2022a) Chronological development of in-patient oncology in times of COVID-19: a retrospective analysis of hospitalized oncology and COVID-19 patients of a German University Hospital. J Cancer Res Clin Oncol 30:30. https://doi.org/10.1007/s00432-022-04044-8
Griewing S, Wagner U, Lingenfelder M, Heinis S, Schieffer B, Markus B et al (2022b) Impact of the COVID-19 pandemic on delivery of gynecology and obstetrics services at a maximum care university hospital in Germany. Geburtshilfe Frauenheilkd 82:427–440. https://doi.org/10.1055/a-1687-9674
Gschnell M, Federspiel P, Wolf R (2021) COVID-19-lockdown impacts medical care-a retrospective analysis of the first wave at a university outpatient clinic in spring 2020. Aktuelle Derm 47:552–557. https://doi.org/10.1055/a-1660-4813
Günster C, Drogan D, Hentschker C, Klauber J, Malzahn J, Schillinger G et al (2020) WIdO-report: entwicklung der Krankenhausfallzahlen Während des coronavirus-lockdowns (report). Wissenschaftliches Institut Der AOK (WIdO). https://doi.org/10.4126/FRL01-006421684. Accessed 26 Oct 2022
Haier J, Beller J, Adorjan K, Bleich S, De Greck M, Griesinger F et al (2022a) Decision conflicts in clinical care during COVID-19: a patient perspective. Healthcare 10:31. https://doi.org/10.3390/healthcare10061019
Haier J, Beller J, Adorjan K, Bleich S, de Greck M, Griesinger F et al (2022b) Decision conflicts in clinical care during Covid-19: a multi-perspective inquiry. Healthcare 10:29. https://doi.org/10.3390/healthcare10101914
Haier J, Beller J, Adorjan K, Bleich S, de Greck M, Griesinger F et al (2022c) Differences in stakeholders’ perception of the impact of COVID-19 on clinical care and decision-making. Cancers. https://doi.org/10.3390/cancers14174317
Hajek A, De Bock F, Huebl L, Kretzler B, König HH (2021) Determinants of postponed cancer screening during the covid-19 pandemic: Evidence from the nationally representative covid-19 snapshot monitoring in Germany (cosmo). Risk Manag Healthc Policy 14:3003–3011. https://doi.org/10.2147/RMHP.S297326
Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E et al (2020) Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 371:m4087. https://doi.org/10.1136/bmj.m4087
Harke NN, Radtke JP, Hadaschik BA, Bach C, Berger FP, Blana A et al (2020) To defer or not to defer? A German longitudinal multicentric assessment of clinical practice in urology during the COVID-19 pandemic. PLoS ONE 15:e0239027. https://doi.org/10.1371/journal.pone.0239027
Harke NN, Wagner C, Hermann RM, Hadaschik BA, Radtke JP, Altay-Langguth A et al (2022) Lessons learned after one year of COVID-19 from a urologist and radiotherapist view: a German survey on prostate cancer diagnosis and treatment. PLoS ONE 17:e0269827. https://doi.org/10.1371/journal.pone.0269827
Heidemann C, Paprott R, Huebl L, Scheidt-Nave C, Reitzle L (2020) Selbst eingeschätzte medizinische Versorgung im Verlauf der SARS-CoV-2-Pandemie in Deutschland: Ergebnisse der COSMO-Studie (report). Robert-Koch Institute. Doi: https://doi.org/10.25646/7208.2. Accessed 26 Oct 2022
Heidt V, Knauf W, Illmer T, Engel E, Goetzenich A (2021) Hämatoonkologische Praxen: Trotz Pandemie ambulant gut versorgt (magazine article). Dtsch Arztebl 118:A310–3. www.aerzteblatt.de/lit0621. Accessed 26 Oct 2022
Heimes D, Müller LK, Schellin A, Naujokat H, Graetz C, Schwendicke F et al (2021) Consequences of the COVID-19 pandemic and governmental containment policies on the detection and therapy of oral malignant lesions—a retrospective, multicenter cohort study from Germany. Cancers. https://doi.org/10.3390/cancers13122892
Hermes-Moll K, Walawgo T, Richter M, Osburg S, Hempler I, Blattert L et al (2021) Ambulante Versorgung von Krebserkrankten: Umfrage unter hämatoonkologischen Schwerpunktpraxen zur COVID-19-Lage (magazine article). InFo Hämatologie + Onkologie 29:68–72. https://www.springermedizin.de/covid-19/impfungen/umfrage-unter-haematoonkologischen-schwerpunktpraxen-zur-covid-1/19657926. Accessed 26 Oct 2022
Holzel D, Schubert-Fritschle G, Engel J (2022) Estimation of the risk of progression of breast cancer after the COVID-19 lockdown. Dtsch Arztebl Int 119:368–369. https://doi.org/10.3238/arztebl.m2022.0165
Hunger R, König V, Stillger R, Mantke R (2022) Impact of the COVID-19 pandemic on delays in surgical procedures in Germany: a multi-center analysis of an administrative registry of 176,783 patients. Patient Saf Surg. https://doi.org/10.1186/s13037-022-00331-y
Jacob L, Loosen SH, Kalder M, Luedde T, Roderburg C, Kostev K (2021) Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers 13:1–11. https://doi.org/10.3390/cancers13030408
Jacob L, Kalder M, Kostev K (2022) Decrease in the number of patients diagnosed with cancer during the COVID-19 pandemic in Germany. J Cancer Res Clin Oncol 148:3117–3123. https://doi.org/10.1007/s00432-022-03922-5
Jones D, Neal RD, Duffy SRG, Scott SE, Whitaker KL, Brain K (2020) Impact of the COVID-19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. Lancet Oncol 21:748–750. https://doi.org/10.1016/s1470-2045(20)30242-4
Jördens MS, Loosen SH, Seraphin T, Luedde T, Kostev K, Roderburg C (2021) Impact of the COVID-19 pandemic on consultations and diagnoses in gastroenterology practices in Germany. Front Med. https://doi.org/10.3389/fmed.2021.684032
Joung RH, Nelson H, Mullett TW, Kurtzman SH, Shafir S, Harris JB et al (2022) A national quality improvement study identifying and addressing cancer screening deficits due to the COVID-19 pandemic. Cancer 128:2119–2125. https://doi.org/10.1002/cncr.34157
Justenhoven C, Rieger B (2022) The impact of the corona pandemic on reported data relating to cancer diagnoses, therapy, and follow-up: analyses from the rhineland-palatinate cancer registry. Dtsch Arztebl Int 119:724–731. https://doi.org/10.3238/arztebl.m2022.0299
Kaltofen T, Hagemann F, Harbeck N, Wuerstlein R, Kost BP, Burges A et al (2022) Changes in gynecologic and breast cancer diagnoses during the first wave of the COVID-19 pandemic: analysis from a tertiary academic gyneco-oncological center in Germany. Arch Gynecol Obstet 305:713–718. https://doi.org/10.1007/s00404-021-06211-7
Kapsner LA, Kampf MO, Seuchter SA, Gruendner J, Gulden C, Mate S et al (2020) reduced rate of inpatient hospital admissions in 18 german university hospitals during the COVID-19 lockdown. Front Public Health 8:594117. https://doi.org/10.3389/fpubh.2020.594117
Kirchberg J, Rentsch A, Klimova A, Vovk V, Hempel S, Folprecht G et al (2021) Influence of the First Wave of the COVID-19 Pandemic on Cancer Care in a German Comprehensive Cancer Center. Front Public Health 9:750479. https://doi.org/10.3389/fpubh.2021.750479
Kleemann J, Meissner M, Ozistanbullu D, Balaban U, Old O, Kippenberger S et al (2022) Impact of the Covid-19 pandemic on melanoma and non-melanoma skin cancer inpatient treatment in Germany-a nationwide analysis. J Eur Acad Dermatol Venereol 36:1766–1773. https://doi.org/10.1111/jdv.18217
Klinische Krebsregister Sachsen (2022) Jahresbericht der klinischen Krebsregister in Sachsen 2011 – 2020 (report). https://www.krebsregister-sachsen.de/fileadmin/user_upload/dokumente/auswertungen/2022-07-06_Jahresbericht_KKR_Sachsen.pdf. Accessed 26 Oct 2022
Klinisches Krebsregister für Brandenburg und Berlin (2022a) Auswirkungen der COVID-19-Pandemie auf Diagnose und Therapie des Mammakarzinoms (magazine article). Brandenburgisches Ärzteblatt, Landesärztekammer Brandenburg. https://www.laekb.de/documents/183A20B72FC.pdf. Accessed 26 Oct 2022
Klinisches Krebsregister für Brandenburg und Berlin (2022b) Erste virtuelle Qualitätskonferenz des KKRBB zum Lungenkarzinom (report). https://kkrbb.de/erste-virtuelle-qualitaetskonferenz-des-kkrbb-zum-lungenkarzinom/. Accessed 26 Oct 2022
Kourtidis S, Munst J, Hofmann VM (2022) Effects of the COVID-19 pandemic on head and neck cancer stage and treatment duration. Cureus. 14:e26744. https://doi.org/10.7759/cureus.26744
Kuhlen R, Schmithausen D, Winklmair C, Schick J, Scriba P (2020) The effects of the COVID-19 pandemic and lockdown on routine hospital care for other illnesses. Dtsch Arztebl Int 117:488–489. https://doi.org/10.3238/arztebl.2020.0488
Mangiapane S, Zhu L, Kretschmann J, Czihal T, von Stillfried D (2021) Veränderung der vertragsärztlichen Leistungsinanspruchnahme während der COVID-Krise-Tabellarischer Trendreport für das Jahr 2020 (report). Zentralinstitut für die kassenärztliche Versorgung in der Bundesrepublik Deutschland (Zi). https://www.zi.de/fileadmin/Downloads/Service/Publikationen/Trendreport_4_Leistungsinanspruchnahme_COVID_2021-04-19.pdf. Accessed 26 Oct 2022
Mangiapane S, Zhu L, Kretschmann J, Czihal T, von Stillfried D (2022) Veränderung der vertragsärztlichen Leistungsinanspruchnahme während der COVID-Krise – Tabellarischer Trendreport bis zum Ende des Jahres 2021 (report). Zentralinstitut für die kassenärztliche Versorgung in der Bundesrepublik Deutschland (Zi). https://www.zi.de/fileadmin/Downloads/Service/Publikationen/Zi-TrendReport_2021-Q4_2022-06-10.pdf. Accessed 26 Oct 2022
Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R et al (2020) The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 21:1023–1034. https://doi.org/10.1016/s1470-2045(20)30388-0
Matuschek C, Fischer JC, Combs SE, Fietkau R, Corradini S, Zanker K et al (2020) Measures of infection prevention and incidence of SARS-CoV-2 infections in cancer patients undergoing radiotherapy in Germany, Austria and Switzerland. Strahlenther Onkol 196:1068–1079. https://doi.org/10.1007/s00066-020-01681-1
Medenwald D, Brunner T, Christiansen H, Kisser U, Mansoorian S, Vordermark D et al (2022) Shift of radiotherapy use during the first wave of the COVID-19 pandemic? an analysis of German inpatient data. Strahlenther Onkol 198:334–345. https://doi.org/10.1007/s00066-021-01883-1
Micek A, Diehl K, Teuscher M, Schaarschmidt ML, Sasama B, Ohletz J et al (2022) Melanoma care during one year pandemic in Berlin: decreasing appointment cancellations despite increasing COVID-19 concern. J Dtsch Dermatol Ges 20:962–978. https://doi.org/10.1111/ddg.14799
Michalowsky B, Hoffmann W, Bohlken J, Kostev K (2021) Effect of the COVID-19 lockdown on disease recognition and utilisation of healthcare services in the older population in Germany: a cross-sectional study. Age Ageing 50:317–325. https://doi.org/10.1093/ageing/afaa260
Mostert C, Hentschker, C., Scheller-Kreinsen, D., Günster, C., Malzahn J, Klauber J (2021) Auswirkungen der Covid-19-Pandemie auf die Krankenhausleistungen im Jahr 2020. Krankenhaus-Report 2021 (report). Doi: https://doi.org/10.1007/978-3-662-62708-2_16. Accessed 26 Oct 2022
Piontek D, Klagges S, Schubotz B, Werner C, Wulff J (2021) documented new cases of cancer in the clinical cancer registries of the german state of saxony during the COVID-19 pandemic. Dtsch Arztebl Int 118:328–329. https://doi.org/10.3238/arztebl.m2021.0216
Pollock D, Peters MDJ, Khalil H, McInerney P, Alexander L, Tricco AC et al (2022) Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid Synth. https://doi.org/10.11124/jbies-22-00123
Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ (2016) The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 160:17–28. https://doi.org/10.1007/s10549-016-3960-3
Reichardt P, Bollmann A, Hohenstein S, Glass B, Untch M, Reichardt A et al (2021) decreased incidence of oncology admissions in 75 helios hospitals in Germany during the COVID-19 pandemic. Oncol Res Treat 44:71–75. https://doi.org/10.1159/000512935
Riemann S, Speck I, Gerstacker K, Becker C, Knopf A (2021) Collateral damage of the COVID-19 pandemic: an alarming decline in critical procedures in otorhinolaryngology in a German university hospital. Eur Arch Oto-Rhino-L 278:3417–3423. https://doi.org/10.1007/s00405-020-06519-1
Rückher J, Mangiapane, S., Seufferlein, T., , Pflüger M, Wesselmann, S. (2022) Auswirkungen der Covid-19-Pandemie auf die onkologische Versorgung. Krankenhaus-Report 2022 (report). https://doi.org/10.1007/978-3-662-64685-4_6. Accessed 26 Oct 2022
Rupa R, Sass B, Morales Lema MA, Nimsky C, Voellger B (2020) the demand for elective neurosurgery at a German University hospital during the first wave of COVID-19. Healthcare 8:13. https://doi.org/10.3390/healthcare8040483
Scheidt-Nave C, Barnes B, Beyer AK, Busch MA, Hapke U, Heidemann C et al (2021) care for the chronically ill in Germany-the challenges during the COVID-19 pandemic. J Health Monit 5:2–27. https://doi.org/10.25646/7168
Schuz J, Borkhardt A, Bouaoun L, Erdmann F (2022) The impact of the COVID-19 pandemic on the future incidence of acute lymphoblastic leukaemia in children: projections for Germany under a COVID-19 related scenario. Int J Cancer 151:153–155. https://doi.org/10.1002/ijc.33992
Stang A, Kuhling L, Khil L, Kajuter H, Schutzendubel A, Mattauch V (2020) Drop in cancer reporting by pathologists in north rhine-westphalia, germany, during the COVID-19 lockdown. Dtsch Arztebl Int 117:886–887. https://doi.org/10.3238/arztebl.2020.0886
Stos C, Steffani M, Kohlhaw K, Rudroff C, Staib L, Hartmann D et al (2020) The COVID-19 pandemic: impact on surgical departments of non-university hospitals. BMC Surg 20:313. https://doi.org/10.1186/s12893-020-00970-x
Struck JP, Schnoor M, Schulze A, Hupe MC, Ozimek T, Oppolzer IA et al (2022) Impact of COVID-19 crisis on medical care of patients with metastasized uro-oncologic disease under systemic cancer therapy: a multicenter study in German university hospitals. World J Urol 40:409–418. https://doi.org/10.1007/s00345-021-03868-2
Tang LV, Hu Y (2020) Poor clinical outcomes for patients with cancer during the COVID-19 pandemic. Lancet Oncol 21:862–864. https://doi.org/10.1016/s1470-2045(20)30311-9
Teuscher M, Diehl K, Schaarschmidt ML, Weilandt J, Sasama B, Ohletz J et al (2022) Effects of the COVID-19 pandemic on care of melanoma patients in Berlin, Germany: the Mela-COVID survey. Eur J Dermatol 31:521–529. https://doi.org/10.1684/ejd.2021.4098
The Lancet Oncology (2020) COVID-19: global consequences for oncology. Lancet Oncol 21:467. https://doi.org/10.1016/s1470-2045(20)30175-3
Tillmanns H, Schillinger G, Dräther H (2022) WIdO-Report: Inanspruchnahme von Früherkennungsleistungen der gesetzlichen Krankenversicherung durch AOK-Versicherte im Erwachsenenalter (2009 bis 2020; report). Wissenschaftliches Institut Der AOK (WIdO). https://doi.org/10.4126/FRL01-006431137. Accessed 26 Oct 2022
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D et al (2018) PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/m18-0850
Verma R, Kilgour HM, Haase KR (2022) The psychosocial impact of COVID-19 on older adults with cancer: a Rapid Review. Curr Oncol 29:589–601. https://doi.org/10.3390/curroncol29020053
Voigtlander S, Hakimhashemi A, Inwald EC, Ortmann O, Gerken M, Klug SJ et al (2021) The impact of the COVID-19 Pandemic on cancer incidence and treatment by cancer stage in bavaria, Germany. Dtsch Arztebl Int 118:660–661. https://doi.org/10.3238/arztebl.m2021.0329
von Dercks N, Seehofer D, Steinert M, Krämer S, Branzan D, Dietrich A et al (2020) How severe is the effect of the coronavirus pandemic on the department of surgery of a university hospital? : An analysis of the first 7 weeks. Chirurg 91:755–761. https://doi.org/10.1007/s00104-020-01255-y
Vu E, Schröder C, Dülk J, Stelmes JJ, Vu J, Schilling J et al (2022) Nationwide survey of german outpatient gynecologic oncology practices during the coronavirus disease 2019 pandemic: reactions to the first wave and future perspectives. Breast Care 17:257–263. https://doi.org/10.1159/000518858
Walter J, Sellmer L, Kahnert K, Zauber R, Syunyaeva Z, Kauffmann-Guerrero D et al (2021) Daily routine and access to care: initial patient reported experiences at a german lung cancer center during the COVID-19 pandemic. Respiration 100:90–92. https://doi.org/10.1159/000513849
Walter J, Sellmer L, Kahnert K, Kiefl R, Syunyaeva Z, Kauffmann-Guerrero D et al (2022) Consequences of the COVID-19 pandemic on lung cancer care and patient health in a German lung cancer center: results from a cross-sectional questionnaire. Respir Res 23:18. https://doi.org/10.1186/s12931-022-01931-z
Wang R, Helf C, Tizek L, Neuhauser R, Eyerich K, Zink A et al (2020) The impact and consequences of SARS-CoV-2 pandemic on a single university dermatology outpatient clinic in Germany. Int J Environ Res Public Health 17:26. https://doi.org/10.3390/ijerph17176182
Weisel KC, Morgner-Miehlke A, Petersen C, Fiedler W, Block A, Schafhausen P et al (2020) Implications of SARS-CoV-2 Infection and COVID-19 crisis on clinical cancer care: report of the university cancer center hamburg. Oncol Res Treat 43:307–313. https://doi.org/10.1159/000508272
Wissenschaftliches Institut der AOK (WIdO) (2021) WIdO-Analyse: Auch in der dritten Pandemiewelle wieder Fallzahlrückgänge in den Krankenhäusern (press release). https://zmail.wido.de/news-presse/pressemitteilungen/2021/wido-analyse-auch-in-der-dritten-pandemiewelle-wieder-fallzahlrueckgaenge-in-den-krankenhaeusern/. Accessed 26 Oct 2022
Wissenschaftliches Institut der AOK (WIdO) (2022) Erneut starke Einbrüche bei Darmkrebs-Operationen in der Omikron-Welle (press release). https://al.wido.de/news-presse/pressemitteilungen/2022/erneut-starke-einbrueche-bei-darmkrebs-operationen-in-der-omikron-welle/?L=0. Accessed 26 Oct 2022
Ziegler E, Hill J, Lieske B, Klein J, Dem OVK, Kofahl C (2022) Empowerment in cancer patients: Does peer support make a difference? a systematic review. Psychooncology 31:683–704. https://doi.org/10.1002/pon.5869
Zok K (2021) Gesundheitsverhalten und Erfahrungen mit der ambulantärztlichen Versorgung während der Covid-19-Pandemie - Ergebnisse einer bundesweiten Repräsentativbefragung (report). Wissenschaftliches Institut der AOK (WIdO). https://www.wido.de/publikationen-produkte/widomonitor/widomonitor-2-2021/?L=0. Accessed 26 Oct 2022
Acknowledgements
We thank Mia Reimer for her assistance with this project, Katharina Heldt for performing the search in Embase, and Dr. Susanne Hepe for her feedback on data items.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by a research grant from the Robert Koch Institute (RKI), Berlin, Germany (grant number: 5053837–2.1.2). RKI is a German government organization responsible for disease control and prevention (https://www.rki.de).
Author information
Authors and Affiliations
Contributions
Project conceptualization: KKDS, SH, BB, KK, MI, RM-E, HZ, Methodology: KKDS, SH, BB, KK, MI, RM-E, HZ, Data collection: KKDS, SH, BB, MI, RM-E, MK, AQ, JS, LS, LC, Data processing, analysis and visualization: KKDS, SH, BB, MI, RM-E, MK, AQ, JS, LS, Manuscript first draft: KKDS, SH, MI, Manuscript editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
De Santis, K.K., Helmer, S., Barnes, B. et al. Impact of the COVID-19 pandemic on oncological care in Germany: rapid review. J Cancer Res Clin Oncol 149, 14329–14340 (2023). https://doi.org/10.1007/s00432-023-05063-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05063-9