Abstract
Background
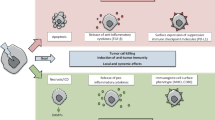
Strategies that restore the immune system's ability to recognize malignant cells have yielded clinical benefits but only in some patients. Tumor cells survive cryotherapy and produce a vast amount of antigens to trigger innate and adaptive responses. However, because tumor cells have developed immune escape mechanisms, cryotherapy alone may not be enough to induce a significant immune response.
Methods
The mice were randomly divided into four groups: Group A: low-dose total body irradiation combined with cryotherapy (L-TBI+cryo); Group B: cryotherapy (cryo); Group C: low-dose total body irradiation(L-TBI); Group D: control group (Control). The tumor growth, recurrence, and survival time of mice in each group were compared and the effects of different treatments on systemic anti-tumor immunity were explored.
Results
L-TBI in conjunction with cryotherapy can effectively control tumor regrowth, inhibit tumor lung metastasis, extend the survival time of mice, and stimulate a long-term protective anti-tumor immune response to resist the re-challenge of tumor cells. The anti-tumor mechanism of this combination therapy may be related to the stimulation of inflammatory factors IFN-γ and IL-2, as well as an increase in immune effector cells (CD8+ T cells) and a decrease in immunosuppressive cells (MDSC, Treg cells) in the spleen or tumor tissue.
Conclusions
We present unique treatment options for enhancing the immune response caused by cryotherapy, pointing to the way forward for cancer treatment.







Similar content being viewed by others
Data availability
Data will be made available on request.
References
Abdo J et al (2018) Immunotherapy plus cryotherapy: potential augmented abscopal effect for advanced cancers. Front Oncol 8:85
Annen R et al (2022) Tumor-specific immunoenhancing effects after local cryoablation for metastatic bone tumor in a mouse model. Int J Mol Sci 23(16):9445
Bae J et al (2022) IL-2 delivery by engineered mesenchymal stem cells re-invigorates CD8 T cells to overcome immunotherapy resistance in cancer. Nat Cell Biol 24(12):1754–1765
Baselet B et al (2019) Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci 76(4):699–728
Baust J, Gage A (2005) The molecular basis of cryosurgery. BJU Int 95(9):1187–1191
Baust J et al (2019) Cryoablation: physical and molecular basis with putative immunological consequences. Int J Hyperth 36:10–16
Burke J, Young H (2019) IFN-γ: a cytokine at the right time, is in the right place. Semin Immunol 43:101280
Chen Z et al (2023) Progress in the cryoablation and cryoimmunotherapy for tumor. Front Immunol 14:1094009
Davoodzadeh Gholami M et al (2017) Exhaustion of T lymphocytes in the tumor microenvironment: Significance and effective mechanisms. Cell Immunol 322:1–14
De Cicco P, Ercolano G, Ianaro A (2020) The new era of cancer immunotherapy: targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol 11:1680
den Brok M et al (2004) In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Can Res 64(11):4024–4029
den Brok M et al (2006) Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 95(7):896–905
Erinjeri J, Clark T (2010) Cryoablation: mechanism of action and devices. J Vasc Interv Radiol 21:S187–S191
Fine R et al (2021) Cryoablation without excision for low-risk early-stage breast cancer: 3-year interim analysis of ipsilateral breast tumor recurrence in the ICE3 trial. Ann Surg Oncol 28(10):5525–5534
Gabrilovich D (2017) Myeloid-derived suppressor cells. Cancer Immunol Res 5(1):3–8
Gerber S et al (2013) IFN-γ mediates the antitumor effects of radiation therapy in a murine colon tumor. Am J Pathol 182(6):2345–2354
Holmes L et al (2001) A rapid, novel strategy to induce tumor cell-specific cytotoxic T lymphocyte responses using instant dentritomas. J Immunother 24(2):122–129
Idriss H, Naismith J (2000) TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc Res Tech 50(3):184–195
Ikeda H, Old L, Schreiber R (2002) The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev 13(2):95–109
Janiak M et al (2017) Cancer immunotherapy: how low-level ionizing radiation can play a key role. Cancer Immunol Immunother 66(7):819–832
Jorgovanovic D et al (2020) Roles of IFN-γ in tumor progression and regression: a review. Biomarker Research 8:49
Katzman D, Wu S, Sterman D (2018) Immunological aspects of cryoablation of non-small cell lung cancer: a comprehensive review. J Thorac 13(5):624–635
Kepp O et al (2020) Oncolysis without viruses-inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol 17(1):49–64
Kim C (2009) FOXP3 and its role in the immune system. Adv Exp Med Biol 665:17–29
Kim K et al (1999) Protective antitumor activity through dendritic cell immunization is mediated by NK cell as well as CTL activation. Arch Pharmacal Res 22(4):340–347
Kojima S et al (2002) Elevation of glutathione induced by low-dose gamma rays and its involvement in increased natural killer activity. Radiat Res 157(3):275–280
Kumar V et al (2016) The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 37(3):208–220
Lin Y et al (2023) Feasibility of computed tomography-guided percutaneous renal cryoablation under local anesthesia: a single center experience in Taiwan. Anticancer Res 43(4):1699–1708
Liu R et al (2010) Enhancement of antitumor immunity by low-dose total body irradiationis associated with selectively decreasing the proportion and number of T regulatory cells. Cell Mol Immunol 7(2):157–162
Liu J et al (2019) Low-dose total body irradiation can enhance systemic immune related response induced by hypo-fractionated radiation. Front Immunol 10:317
Mahnken A, König A, Figiel J (2018) Current technique and application of percutaneous cryotherapy. Fortschr Röntgenstr 190(9):836–846
Miwa S et al (2012) TNF-α and tumor lysate promote the maturation of dendritic cells for immunotherapy for advanced malignant bone and soft tissue tumors. PLoS One 7(12):e52926
Nishikawa H, Koyama S (2021) Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer 9(7):e002591
Pandey R et al (2005) Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. Int J Radiat Biol 81(11):801–812
Parker K, Beury D, Ostrand-Rosenberg S (2015) Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res 128:95–139
Pulaski B, Ostrand-Rosenberg S (2001) Mouse 4T1 breast tumor model. Curr Protoc Immunol. https://doi.org/10.1002/0471142735.im2002s39
Safwat A (2000) The immunobiology of low-dose total-body irradiation: more questions than answers. Radiat Res 153:599–604
Sampadi B et al (2022) Divergent molecular and cellular responses to low and high-dose ionizing radiation. Cells 11(23):3794
Shimizu K et al (2018) Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol 30(10):445–454
Takeda K et al (2001) Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell Immunol 214(2):194–200
Tariq R et al (2020) Efficacy of cryotherapy as a primary endoscopic ablation modality for dysplastic Barrett’s esophagus and early esophageal neoplasia: a systematic review and meta-analysis. Cancer Control 27(1):1073274820976668
Thomsen L et al (2023) A phase I prospective, non-randomized trial of autologous dendritic cell-based cryoimmunotherapy in patients with metastatic castration-resistant prostate cancer. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-023-03421-7
Tian Y et al (2022) Cryoablation and immune synergistic effect for lung cancer: a review. Front Immunol 13:950921
Wu Y et al (2022) Cryoablation reshapes the immune microenvironment in the distal tumor and enhances the anti-tumor immunity. Front Immunol 13:930461
Wülfing C, Günther H (2015) Dendritic cells and macrophages neurally hard-wired in the lymph node. Sci Rep 5:16866
Yang G et al (2014) Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother Radiopharm 29(10):428–434
Yang C et al (2018) Ki67 targeted strategies for cancer therapy. Clin Transl Oncol 20(5):570–575
Zheng X et al (2015) Recovery profiles of T-cell subsets following low-dose total body irradiation and improvement with cinnamon. Int J Radiat Oncol Biol Phys 93(5):1118–1126
Acknowledgements
The authors would like to express their appreciation to appreciate Department of Oncology and Department of Pathology, Affiliated Hospital of Southwest Medical University, Luzhou, China. We appreciate all coresearchers for their continuous collaborations with us in sharing information.
Funding
This work was supported by grants from the Union Project of Luzhou City and the Southwest Medical University (Nos. 14JC0144, 2013LZLY-J40).
Author information
Authors and Affiliations
Contributions
YL, YC and SL performed experiments, analyzed the data. All authors contributed to write, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All animal experiments were approved by the Experimental Animal Ethics Committee of Southwest Medical University, and each mouse was treated humanely.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, Y., Chen, Y., Liu, S. et al. Low-dose total body irradiation enhances systemic anti-tumor immunity induced by local cryotherapy. J Cancer Res Clin Oncol 149, 10053–10063 (2023). https://doi.org/10.1007/s00432-023-04928-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04928-3