Abstract
Purpose
It was of great significance to identify someone with a high risk of hepatocellular carcinoma (HCC) occurrence and make a diagnosis as early as possible. Therefore, we aimed to develop and validate a new, objective, and accurate prediction model, and convert it into a nomogram to make a personalized prediction of cancer occurrence in cirrhotic patients with different etiologies.
Methods
The present study included 938 patients with cirrhosis from January 1, 2011, to December 31, 2012. Patients were prospectively followed-up until January 1, 2018. We used a competing risk model and the Fine–Gray test to develop and validate the prediction model and to plot a nomogram based on the model established.
Results
At the end of follow-up, 202 (21.5%) patients developed HCC, with a 5-year incidence of 19.0% (corrected for competing risk model). Based on the competing risk regression method, we built a prediction model including age, gender, etiology, lymphocyte, and A/G ratio. Three groups with different risks were generated on account of tertiles of the 5-year risk predicted by the model. The cumulative 1-, 3-, and 5-year incidences of HCC were 2.0%, 20.8%, and 42.3% in high-risk group, 0.9%, 10.1%, and 17.7% in medium-risk group, and 0%, 2.0%, 8.5% in low-risk group (P < 0.001). The model showed excellent discrimination and calibration in predicting the risk of HCC occurrence in patients with all-cause cirrhosis.
Conclusion
The model could make an individual prediction of cancer occurrence and stratify patients based on predicted risk, regardless of the causes of cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Implications for practice
To improve long-term survival in patients with HCC, it is urgent to identify individuals with high risk of cancer occurrence and make a diagnosis as early as possible. Existing models are established based on Cox proportional hazards model, and these studies do not take competing risk events into account. Using the competing risk model, we developed and validate a new prediction tool. Our model could screen out patients with low risk of cancer occurrence, who could receive less intensive cancer surveillance. In contrast, for patients with the high cancer risk, enhanced follow-up was recommended for screening and diagnosis of HCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of tumor-related death worldwide (Sung et al. 2021). The prognosis of patients with different tumor stages varies significantly, with a 5-year overall survival (OS) rate of 70–75% in the early stage, while with an average survival time of less than 12 months in the advanced stage (Llovet and Bruix 2000; Ioannou et al. 2008; Villanueva 2019). It is reported that the 5-year OS rate of liver cancer in China is only about 12.1% (Zeng et al. 2018). This is mainly due to the low rate of early diagnosis, that is, most patients have advanced tumors when they are diagnosed (Trinchet et al. 2011). As a routine monitoring technique, ultrasound has poor sensitivity for small tumors (< 2 cm), which can be accurately diagnosed by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) (Singal et al. 2014). However, these expensive imaging technologies are not cost-effective for someone with cirrhosis who are at low risk of cancer occurrence (Kim et al. 2017; Cadier et al. 2017).
To improve long-term survival in patients with HCC, it is urgent to identify individuals with a high risk of cancer occurrence and make a diagnosis as early as possible. So far, several models such as PAGE-B, mPAGE-B, REAL-B, THRI, and so on have been developed and validated to assess the occurrence risk of liver cancer (Yang et al. 2020; Kim et al. 2018; Hu et al. 2020; Papatheodoridis et al. 2016; Ioannou et al. 2018, 2019; Yu et al. 2019; Sharma et al. 2017). The cases of non-viral-related liver diseases like alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) are increasing year by year, which is endangering human life and health. However, most of these models are based on selected patients with hepatitis B virus- (HBV) or hepatitis C virus- (HCV) associated liver disease, with a condition of antiviral treatment. Therefore, the generalization of the models in patients with all-cause cirrhosis is somewhat limited (Heimbach et al. 2018). From the perspective of statistical methods, all these models are established based on Cox proportional hazards model, and these studies do not take competing risk events into account. For Cox proportional hazards model, death is treated as a censoring event, that is to say, it is believed that cancer occurrence will be observed in the case of continued follow-up. Whereas this is not the case, and Cox regression often overestimates the cumulative incidence risk (Putter et al. 2007; Berry et al. 2010).
In conclusion, using the competing risk model, we aimed to develop and validate a new prediction tool that was more consistent with the real-world association model, and convert it into a nomogram to make an individualized prediction of cancer risk in patients with all-cause cirrhosis in this large and prospective cohort study.
Methods
Patients enrolled
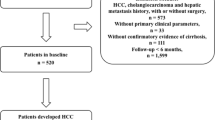
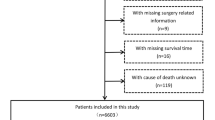
A total of 938 patients with all-cause cirrhosis who were admitted to Beijing You’an Hospital affiliated with Capital Medical University from January 1, 2011, to December 31, 2012, were enrolled. All patients were diagnosed with cirrhosis by imaging and histological examination based on etiology, medical history, clinical manifestation, and complications. Generally speaking, splits by different hospitals or by admission time were both attractive approaches to develop and externally validate a prediction model, whereas our study divided the data set into derivation cohort and external validation cohorts by the time of visit (known as temporal validation) (Moons et al. 2012; Steyerberg and Harrell 2016). Therefore, to improve the generalization capability and external applicability of the model, 457 patients treated in 2011 were included in the derivation cohort, and 481 patients treated in 2012 were included in the validation cohort for temporal validation.
Clinicopathological data
Thirty-four indicators, including demographic and baseline clinicopathological data, were collected and summarized as follows: (1) demographic data: age, gender, the history of antiviral, hypertension and diabetes mellitus; (2) etiology of cirrhosis: HBV, HCV, ALD, co-infection, and others (NAFLD, primary biliary cirrhosis, autoimmune hepatitis, drug-induced liver injury, Budd–Chiari syndrome, etc.); (3) blood routine examination: white blood cell, neutrophil, lymphocyte, monocyte, hemoglobin, platelet; (4) liver and renal function examination: Child–Pugh class; alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), total protein (TP), albumin, globulin, γ-glutamyl transpeptidase (γ-GT), alkaline phosphatase (ALP), prealbumin, total bile acid (TBA), cholinesterase (ChE), cholesterol; (5) coagulation markers: prothrombin time (PT), prothrombin time activity (PTA), international normalized ratio (INR), fibrinogen, activated partial thromboplastin time (APTT), thrombin time (TT); (6) other indicators: alpha fetoprotein (AFP) and viral load.
Compared with albumin or globulin alone, the albumin-to-globulin ratio (A/G ratio) is not easily affected by changes in body fluids, such as hemoconcentration or hemodilution. In modeling, therefore, we included the A/G ratio, which can be used as a more objective and stable clinical parameter to assess the risk of cancer occurrence in patients with cirrhosis.
Follow-up
The enrolled patients in both the derivation and validation cohorts were followed-up every 6 months in the outpatient clinic, including medical examination, laboratory, and ultrasonic examination. Once focal lesions were reported, contrast-enhanced CT or MRI and/or histological examination were performed immediately for definitive diagnosis in accordance with the diagnostic procedures recommended by the AALSD guidelines (Heimbach et al. 2018). Therefore, follow-up strategies were consistent between the derivation and validation cohorts. Events (e.g., death, cause of death, occurrence of hepatocellular carcinoma, liver transplantation) during follow-up were recorded in detail. The process of recording information was monitored by three Clinical Research Associates (LJJ, ZYH, and GWF). The medical diagnosis during the follow-up was confirmed by two senior hepatologists with 10 years of experience in our center (ZYH and ZJS).
Standard of diagnosis
Diagnostic criteria for cirrhosis (one of the following three): (1) The presence of pseudolobule and regenerative nodule is reported on histological examination; (2) endoscopy demonstrates esophageal and gastric varices or ectopic varices, except for non-cirrhotic portal hypertension; (3) the results of ultrasonography, liver stiffness measurement (LSM) or CT suggest the characteristics of cirrhosis or portal hypertension, such as splenomegaly, ascites, hepatic encephalopathy, portal vein diameter not less than 1.3 cm (Ginès et al. 2021). For nodules > 1 cm detected by ultrasound examination, contrast-enhanced CT or MRI is performed. Diagnosis of HCC is made when at least one imaging examination showed significant enhancement in the arterial phase while washout in the portal vein phase and/or delayed phase. For patients with atypical imaging features but suspected malignant nodules reported by CT or MRI, a further needle biopsy is required to confirm the diagnosis (Heimbach et al. 2018).
Statistical analyses
Logarithmic transformation was performed for continuous data that did not conform to the normal distribution. If the data after transformation still did not conform to the normal distribution, the original data would be retained and appropriate statistical methods were selected for analysis. The continuous data conforming to the normal distribution were represented by the means ± standard deviation, and if not, by the median (interquartile range, IQR). Categorical data were expressed as frequency or percentage. Depending on whether the data obeyed the normal distribution, Student's t test or Mann–Whitney U test was used between the two groups, and a one-way ANOVA analysis or Kruskal–Wallis test was performed between the three groups for continuous data. The Chi-square test was used for the different comparison of categorical data. The non-linear relationship between variables and outcome was analyzed using the restricted cubic spline (RCS) method with five knots. The cumulative incidence curve was plotted to assess the time-dependent cumulative incidences of primary endpoint and competing risk events.
In this study, death or liver transplantation (represented by the number 2) would hinder cancer occurrence (represented by the number 1), and there was competing risk between 1 and 2, which were mutually competing risk events. Therefore, a competing risk model was used to screen independent risk factors and establish the model. Variables with a P value less than 0.1 in univariate analysis were included in multivariate analysis. Sub-hazard ratios (SHRs) and 95% confidence interval (CI) were reported, with regression coefficients (log [SHR]) considered as weights to calculate the predicted risk score and plot the nomogram. After the model was established, internal and external validation was carried out based on discrimination, calibration, and clinical value. The Bootstrap sampling method was performed to calculate Harrell's concordance index. Calibration curves were drawn to evaluate the degree of consistency between the predicted and the observed probability. Three groups with different occurrence risks (low-risk, medium-risk, and high-risk) were generated on account of tertiles of the 5-year risk predicted by the model established. The cumulative incidence curves of the three groups were plotted for clinical applicability analysis.
It was difficult to calculate the sample size beforehand due to weak evidence in establishing a risk stratification model for predicting the development of HCC in cirrhotic patients. Nevertheless, the high number of HCC incidences (more than 200) compared with the number of Cox model variables (5) implied that the “ten events per variable” rule was largely exceeded, thus indicating sufficient accuracy and precision of estimates (Peduzzi et al. 1995).
A P value less than 0.05 was considered statistically significant. All statistical analyses were conducted by R software version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics of patients enrolled
A total of 938 patients with all-cause cirrhosis admitted to our hospital from January 1, 2011, to December 31, 2012, were included, with an average age of 51 years. There were 602 cases of HBV, 109 of HCV, 104 of ALD, 9 of HBV/HCV co-infection, and 114 of other etiologies (2 NAFLD, 27 primary biliary cirrhosis, 12 autoimmune hepatitis, 10 drug-induced liver injury, 2 Budd–Chiari syndromes, and 61cryptogenic cirrhosis). There were 645 cases (68.8%) in males, 156 (16.6%) with hypertension, 137 (14.6%) with diabetes mellitus, and 617 (65.8%) with antiviral history. 453 (48.3%) patients were Child–Pugh class A, 308 (32.8%) were class B, and 177 (18.9%) were class C.
We divided patients into three groups (cirrhosis group, cancer group, and competing event group) based on patient status during follow-up, and compared the baseline data of the three groups (Table 1 and Supplementary Fig. 1). Interestingly, among the 34 indicators studied by us, 31 had significant statistical differences (P < 0.05), and the P values of the remaining 3 were critically positive (P = 0.05–0.1). The baseline data of the derivation and validation cohort were compared (Table 2). The results showed that in addition to differences in some parameters (ALT, AST, TBA, etc.), the number of patients with HCC occurrence was higher in the derivation cohort (26.0% versus 17.3%).
Follow-up and patients outcomes
Sixty-eight cases were lost to follow-up with the last follow-up time of January 1, 2018. The median follow-up time was 66.2 months (IQR: 48.4–74.4). Sixteen patients received liver transplantation. By the end of the follow-up, 202 patients developed HCC. The etiologies of 202 HCC patients were summarized as follows: 138 cases of HBV, 24 cases of HCV, 20 cases of ALD, 15 cases of other causes, and 5 cases of co-infection. The cumulative cancer incidences of 1, 3, and 5 years were 1.5% (14/938), 10.1% (95/938), 18.1% (170/938), and 5-year incidence corrected by the competing risk model was 19.0% (Supplementary Fig. 2). The characteristics of HCC are summarized in Supplementary Table 1. When diagnosed, about 70% of patients had single or small tumors, and about 65% had BCLC stage A.
A total of 89 (9.5%) patients died, with a 5-year OS of 92.7%. The causes of death included gastrointestinal bleeding in 27 (30.3%) cases, spontaneous bacterial peritonitis in 23 (25.8%) cases, liver failure in 18 (20.2%), hepatic encephalopathy in 11 (12.4%) cases, multiple organ failure in 4 (4.5%) cases, and others causes in 6 (6.8%) cases (2 hepatorenal syndromes, 2 pulmonary infections, 2 other malignant diseases (1 colorectal cancer, 1 gastric cancer)). Marimekko plots were used to analyze the occurrence of cancer and death among patients with various etiologies and found that patients with virus-associated cirrhosis had the highest incidence of cancer (23.19%) and the lowest mortality (8.75%). Five (55.6%) of nine patients with co-infection had HCC (Fig. 1a). Notably, patients with cirrhosis of other etiologies had the highest mortality (21.93%) and the lowest cancer incidence (13.16%) (Fig. 1b).
Competing risk model for predicting HCC occurrence
The unadjusted univariate analysis and multivariate competing risk regression were performed (Table 3). Univariate analysis showed that 14 indicators including age, gender, etiology, Child–Pugh class, lymphocyte, platelet, A/G ratio, prealbumin, ChE, PT, PTA, INR, AFP, and viral load were associated with increased occurrence risk of HCC. Five independent risk factors, involving male, old age, virus-associated cirrhosis, and low levels of lymphocyte and A/G ratio, were finally identified by multivariate competing risk regression analysis and then incorporated into the model (YOUAN model). Although the P value of lymphocyte was 0.077 in multivariate analysis, it was well known that low lymphocyte was related to poor prognosis of many diseases. Therefore, to improve the performance, lymphocyte was considered in the model, which increased Harrell's concordance index from 0.718 to 0.732.
Prognostic factors analysis based on Fine–Gray test
Cumulative incidence curves were plotted based on gender and etiology (Fig. 2). For gender, after the Fine–Gray test, it could be seen that the cancer risk was a statistical difference between the two groups (Fig. 2a). The cumulative incidences of 1, 3, and 5 years in males were 0.9%, 14.0%, and 24.9%, while 1.5%, 8.4%, and 14.6% in females (P = 0.043). There was no significant difference in the incidence of competing risk events between the two groups. As shown in Fig. 2b, the cumulative 1-, 3-, and 5-year cancer incidences were higher in patients with virus-associated cirrhosis than that with non-virus-associated cirrhosis (1.1%, 13.4, 24.4% versus 1.0%, 8.8%, 12.9%; P = 0.017). However, the corresponding incidences of competing risk events were higher in patients with non-virus-associated cirrhosis than that in the other group (0%, 6.9%, 13.1% versus 0.3%, 2.3%, 5.8%; P = 0.002).
The non-linear relationship between the parameters of age, lymphocyte, and A/G ratio and cancer occurrence based on the RCS method was explored. The results showed that the risk of HCC tended to be stable before 55 years old and increased rapidly after that age (Fig. 3A). Therefore, the cutoff value of 55 was used to divide the patients into two groups and draw the cumulative incidence curves of both groups. Both the cumulative incidences of HCC and competing risk events were higher in patients older than 55 years old (Fig. 3b). The cumulative 1-, 3-, and 5-year cancer incidences were 0%, 9.1%, and 16.1% for patients under 55 years, while 2.8%, 17.5%, and 30.9% for patients over 55 years old, respectively (P < 0.001).
Similarly, we performed the above analysis on lymphocytes and the A/G ratio. The risk of cancer was significantly increased when the absolute value of lymphocyte was less than 0.97 (10^9/L) (Fig. 3C). Then the patients were divided into two groups with a cutoff value of 0.97, and it was found that there was a significant statistical difference in the cumulative 1-, 3-, and 5-year cancer incidences between the two groups (1.6%, 18.4%, 29.0% versus 0.8%, 8.0%, 16.7%; P = 0.004). There was no difference in the incidence of competing risk events (Fig. 3D). For the A/G ratio, the risk of cancer was significantly increased when it was less than 1.18 (Fig. 3E). The cutoff value of 1.18 was used to divide the patients into two groups, and we found that the cumulative 1-, 3-, and 5-year cancer incidences in low A/G ratio group were significantly higher than patients in high A/G ratio group (0.5%, 14.7%, 26.5% versus 1.5%, 10.7%, 18.5%; P = 0.007). There was no significant difference in the cumulative incidence of competing risk events between the two groups (Fig. 3F).
Evaluation of discrimination and calibration of the established model
The model's discrimination (i.e., ability to distinguish those who will develop HCC from those who will not), calibration (i.e., the degree of consistency between the predicted probability by the model and the observed probability), and clinical value was assessed. The Harrell's concordance index of the model was calculated, with 0.732 for the derivation cohort and 0.729 for the validation cohort. Because the 1-year cumulative incidence of HCC was low, calibration curves of predicting 2-, 3-, and 5-year HCC occurrence were drawn in the two cohorts, respectively. It could be seen that the predicted probability was in good agreement with the observed probability (Fig. 4).
Nomogram and analysis of clinical value
The nomogram based on the results of competing risk regression was plotted for clinical application (Fig. 5). For example, a 70-year-old male patient with alcohol-related cirrhosis, and with an A/G ratio of 1.2 and an absolute value of lymphocyte of 1.5 (10^9/L), had a total score of about 18.8, and the corresponding 3- and 5-year cancer incidences were about 12% and 22% (Table 4). To evaluate the model's ability to identify patients with different cancer risks, three groups (low-risk, medium-risk, and high-risk) were generated on account of tertiles of the 5-year risk predicted by the YOUAN model in the validation cohort and the whole cohort, and the cumulative incidence curves of the three groups were plotted, respectively. It was found that the YOUAN model could stratify patients in both the validation cohort and the whole cohort according to the disparate risk of HCC and competing risk events (Fig. 6). For the validation cohort, the cumulative 1-, 3-, and 5-year incidences of HCC were 3.5%, 17.5%, and 33.0% in the high-risk group, 2.0%, 8.9%, and 17.5% in the medium-risk group, and 0%, 4.8%, 7.3% in the low-risk group (P < 0.001). The corresponding incidences in the whole cohort were 2.0%, 20.8%, and 40.3% in high-risk group, 0.9%, 10.1%, and 19.7% in medium-risk group, and 0%, 2.0%, 9.5% in low-risk group (P < 0.001). In addition, patients with a higher risk of cancer had a higher risk of competing risk events, which meant that the YOUAN model could predict death to some extent (Supplementary Fig. 3).
Discussion
In this study, we successfully developed and validated a simple and accurate YOUAN model to predict the risk of HCC based on the competing risk regression, which contains five clinical indicators of routine examination, involving gender, age, etiology of cirrhosis, lymphocyte, and A/G ratio. The model showed excellent discrimination and calibration in assessing the cumulative cancer incidences of 2, 3, and 5 years in both the derivation cohort and validation cohort, regardless of etiologies of cirrhosis. The 5-year incidence of HCC could reach 40% in the high-risk group while less than 10% in the low-risk group. To date, the aMAP score was the first accurate, high-level, and simple-to-use model to predict individualized HCC risk for patients with chronic liver disease in the world, regardless of etiology, ethnicity or antiviral therapy (Fan et al. 2020). Likewise, we also developed the YOUAN model that stratifies patients according to the different risks of cancer, regardless of etiologies of cirrhosis. Therefore, in this study, the predictive performance of the YOUAN model was compared with aMAP score. The Harrell's concordance indexes of the YOUAN model were higher than that of aMAP score in both the derivation cohort (0.732 versus 0.692) and the validation cohort (0.729 versus 0.705).
We found that HCC occurrence was the highest in patients with virus-associated cirrhosis, with more than 50% in patients with co-infection of HBV and HCV, while mortality was the highest in patients with cirrhosis of other causes with the lowest cancer incidence. Both males and patients aged older than 55 had a higher risk of cancer than females and younger patients. Other studies have also reported that age and gender were independent predictors for evaluating the occurrence of liver cancer (Yang et al. 2020; Kim et al. 2018; Papatheodoridis et al. 2016; Ioannou et al. 2018, 2019; Yu et al. 2019; Sharma et al. 2017). For the etiology of cirrhosis, chronic HBV or HCV infection was still the most important cause of liver cancer so far, and the annual incidence of HCC was 2–5% in patients with virus-related cirrhosis (El-Serag 2012; Yang et al. 2016; Yang and Roberts 2010). Alcoholic liver disease was the second most common risk factor for liver cancer (Park et al. 2015). Other chronic liver diseases, such as chronic biliary tract disease and hereditary or metabolic liver disease, could also lead to cirrhosis and further promote the development of cancer, but the proportion of cancer caused by these etiologies was less than 5% to 10% worldwide (Yang and Roberts 2010). The above reports were completely consistent with our studies.
Most notably, the YOUAN model involved two clinical indicators that had not been considered in other models, namely lymphocyte and A/G ratio. Lymphocyte, which played an important role in the immune response, was a major factor in inhibiting cancer progression. As a parameter reflecting the strength of the body's immunity, the reduced number of lymphocytes indicated that the body lacked an effective immune response to tumors (Li et al. 2017; Iseki et al. 2017). Previous studies have revealed the potential relationship between chronic inflammation and cancer and found that inflammatory mediators in cells, such as interleukin—6 (IL-6) and tumor necrosis factor-α (TNF-α), could change the tumor microenvironment and promote the proliferation, malignant transformation, and metastasis of tumor cells (Pfensig et al. 2016; Arroyo et al. 2014). The serum A/G ratio was one of the important markers to reflect systemic inflammation. On the one hand, albumin was related to the nutritional status of patients. Hypoproteinemia in patients meant malnutrition, decreased immunity, and weakened defense ability. On the other hand, inflammatory factors such as IL-6 and TNF-α could affect the synthesis of albumin by hepatocytes, thus increasing the risk of infection and promoting the invasion and metastasis of tumors (Gupta and Lis 2010). Studies have shown that low albumin led to immunosuppression, impaired lymphocyte function, and reduced lymphocyte count (Chen et al. 2015). High levels of globulin could be regarded as a marker of the activated inflammatory response (Macfarlane et al. 2016). The composition of globulin was more complex, including interleukin, C-reaction protein, etc., which play an important role in the occurrence, development, and metastasis of tumors.
There were some limitations to our study. First of all, this was a single-center study. However, we divided the patients into derivation cohort and validation cohort according to the time of visit. Temporal validation, as a type of external validation, could strengthen the transportability and generalization ability of the model. Second, the YOUAN model only included five clinical indicators of routine examination and did not take into account other variables (such as proteins or metabolites, and circulating cell-free DNA signatures). The original intention of this study was to develop an economical and cost-effective prediction model based on routine laboratory indicators for clinical application. Nevertheless, to further optimize the model established, our team will consider combining the above indicators with the existing model in future work. Third, the study was conducted based on an Asian population, limiting predictive power for patients of other races.
Our study had several advantages. First, indicators involved in this study covered a wide range, including 34 variables of demographic data, etiology of cirrhosis, blood routine examination, liver and renal function examinations, coagulation markers, and others. Second, a competing risk model with a consideration of competing risk events was performed. Focusing only on cancer occurrence and ignoring competing risk events would lead to biased estimates of individual incidence. Third, it was the first study to incorporate lymphocyte and the A/G ratio as predictors of HCC occurrence into the model. Some studies included albumin instead of the A/G ratio in their models (Kim et al. 2018; Ioannou et al. 2018, 2019; Yu et al. 2019). However, compared with albumin or globulin alone, the A/G ratio was not easily affected by changes in body fluids, such as hemoconcentration or hemodilution, which could be used as a more objective and stable clinical indicator to assess the risk of cancer in patients. Finally, the etiological profiles of cirrhosis and HCC developing from cirrhosis of different causes were delineated in as much detail as possible. And the YOUAN model was developed based on patients with all-cause cirrhosis, while most other models for a patient population with a specific etiology, such as virus-associated cirrhosis, alcoholic liver disease, non-alcoholic fatty liver disease, etc. (Papatheodoridis et al. 2016; Ioannou et al. 2018, 2019; Alexander et al. 2019). And the YOUAN model could also predict death to some extent.
As an approach to decrease the cost and increase the cost-effectiveness, early diagnosis through the development of a personalized HCC monitoring strategy was still the best solution to improve the possibility of curing liver cancer and reducing mortality. Our model could screen out patients with a low risk of cancer occurrence, who could receive less intensive liver cancer surveillance, thereby saving medical resources. In contrast, for patients with high cancer risk, enhanced follow-up or more accurate but expensive imaging techniques were recommended for screening and diagnosis of HCC.
In response to the ambitious goal of reducing hepatitis-related mortality by 65% by 2030 set by the World Health Organization (WHO) (Mbuagbaw et al. 2017), we developed the “YOUAN model” that stratifies patients according to the different risks of cancer, regardless of etiologies of cirrhosis, which would be an effective and operable tool to improve the early diagnosis of liver cancer and reduce the mortality.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D et al (2019) Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med 17:95. https://doi.org/10.1186/s12916-019-1321-x
Arroyo V, García-Martinez R, Salvatella X (2014) Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol 61:396–407. https://doi.org/10.1016/j.jhep.2014.04.012
Berry SD, Ngo L, Samelson EJ, Kiel DP (2010) Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc 58:783–787. https://doi.org/10.1111/j.1532-5415.2010.02767.x
Cadier B, Bulsei J, Nahon P, Seror O, Laurent A, Rosa I et al (2017) Early detection and curative treatment of hepatocellular carcinoma: a cost-effectiveness analysis in France and in the United States. Hepatology 65:1237–1248. https://doi.org/10.1002/hep.28961
Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K et al (2015) Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget 6:41370–41382. https://doi.org/10.18632/oncotarget.5629
El-Serag HB (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142:1264-1273.e1261. https://doi.org/10.1053/j.gastro.2011.12.061
Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q et al (2020) aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol 73:1368–1378. https://doi.org/10.1016/j.jhep.2020.07.025
Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS (2021) Liver cirrhosis. Lancet 398:1359–1376. https://doi.org/10.1016/s0140-6736(21)01374-x
Gupta D, Lis CG (2010) Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 9:69. https://doi.org/10.1186/1475-2891-9-69
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR et al (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358–380. https://doi.org/10.1002/hep.29086
Hu TH, Yueh-Hsia Chiu S, Tseng PL, Chen CH, Lu SN, Wang JH et al (2020) Five-year comparative risk of hepatocellular carcinoma development under entecavir or tenofovir treatment-naïve patients with chronic hepatitis B-related compensated cirrhosis in Taiwan. Aliment Pharmacol Ther 52:1695–1706. https://doi.org/10.1111/apt.16116
Ioannou GN, Perkins JD, Carithers RL Jr (2008) Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 134:1342–1351. https://doi.org/10.1053/j.gastro.2008.02.013
Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, Berry K (2018) Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol 69:1088–1098. https://doi.org/10.1016/j.jhep.2018.07.024
Ioannou GN, Green P, Kerr KF, Berry K (2019) Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol 71:523–533. https://doi.org/10.1016/j.jhep.2019.05.008
Iseki Y, Shibutani M, Maeda K, Nagahara H, Tamura T, Ohira G et al (2017) The impact of the preoperative peripheral lymphocyte count and lymphocyte percentage in patients with colorectal cancer. Surg Today 47:743–754. https://doi.org/10.1007/s00595-016-1433-2
Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH et al (2017) MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol 3:456–463. https://doi.org/10.1001/jamaoncol.2016.3147
Kim JH, Kim YD, Lee M, Jun BG, Kim TS, Suk KT et al (2018) Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J Hepatol 69:1066–1073. https://doi.org/10.1016/j.jhep.2018.07.018
Li N, Zhang L, Song HL, Zhang J, Weng HW, Zou LQ (2017) Prognostic impact of absolute lymphocyte count/absolute monocyte count ratio and prognostic score in patients with nasal-type, extranodal natural killer/T-cell lymphoma. Tumour Biol 39:1010428317705503. https://doi.org/10.1177/1010428317705503
Llovet JM, Bruix J (2000) Early diagnosis and treatment of hepatocellular carcinoma. Baillieres Best Pract Res Clin Gastroenterol 14:991–1008. https://doi.org/10.1053/bega.2000.0143
Macfarlane L, Morris J, Pratschke K, Mellor D, Scase T, Macfarlane M et al (2016) Diagnostic value of neutrophil-lymphocyte and albumin-globulin ratios in canine soft tissue sarcoma. J Small Anim Pract 57:135–141. https://doi.org/10.1111/jsap.12435
Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L et al (2017) Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev 6:79. https://doi.org/10.1186/s13643-017-0473-z
Moons KG, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG et al (2012) Risk prediction models: II. External validation, model updating, and impact assessment. Heart 98:691–698. https://doi.org/10.1136/heartjnl-2011-301247
Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J et al (2016) PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol 64:800–806. https://doi.org/10.1016/j.jhep.2015.11.035
Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ et al (2015) Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 35:2155–2166. https://doi.org/10.1111/liv.12818
Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48:1503–1510. https://doi.org/10.1016/0895-4356(95)00048-8
Pfensig C, Dominik A, Borufka L, Hinz M, Stange J, Eggert M (2016) A new application for albumin dialysis in extracorporeal organ support: characterization of a putative interaction between human albumin and proinflammatory cytokines IL-6 and TNFα. Artif Organs 40:397–402. https://doi.org/10.1111/aor.12557
Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26:2389–2430. https://doi.org/10.1002/sim.2712
Sharma SA, Kowgier M, Hansen BE, Brouwer WP, Maan R, Wong D et al (2017) Toronto HCC risk index: a validated scoring system to predict 10-year risk of HCC in patients with cirrhosis. J Hepatol. https://doi.org/10.1016/j.jhep.2017.07.033
Singal AG, Pillai A, Tiro J (2014) Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 11:e1001624. https://doi.org/10.1371/journal.pmed.1001624
Steyerberg EW, Harrell FE Jr (2016) Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol 69:245–247. https://doi.org/10.1016/j.jclinepi.2015.04.005
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H et al (2011) Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 54:1987–1997. https://doi.org/10.1002/hep.24545
Villanueva A (2019) Hepatocellular Carcinoma. N Engl J Med 380:1450–1462. https://doi.org/10.1056/NEJMra1713263
Yang JD, Roberts LR (2010) Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol 7:448–458. https://doi.org/10.1038/nrgastro.2010.100
Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR (2016) Diabetes mellitus heightens the risk of hepatocellular carcinoma except in patients with hepatitis C cirrhosis. Am J Gastroenterol 111:1573–1580. https://doi.org/10.1038/ajg.2016.330
Yang HI, Yeh ML, Wong GL, Peng CY, Chen CH, Trinh HN et al (2020) Real-world effectiveness from the Asia Pacific rim liver consortium for HBV risk score for the prediction of hepatocellular carcinoma in chronic hepatitis b patients treated with oral antiviral therapy. J Infect Dis 221:389–399. https://doi.org/10.1093/infdis/jiz477
Yu JH, Suh YJ, Jin YJ, Heo NY, Jang JW, You CR et al (2019) Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis B patients receiving entecavir/tenofovir. Eur J Gastroenterol Hepatol 31:865–872. https://doi.org/10.1097/meg.0000000000001357
Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X et al (2018) Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 6:e555–e567. https://doi.org/10.1016/s2214-109x(18)30127-x
Funding
This study was funded by a grant Beijing Municipal Natural Science Foundation (7191004), Capital health development project (2020–1-2182 and 2020–2-1153), Beijing Key Laboratory (BZ0373), Key medical professional development plan of Beijing municipal administration of hospitals (ZYLX201711), Beijing Municipal Administration of Hospitals’ Incubating Program (PX2018059) and the Beijing You’an Hospital plan (Grant No. YNKTQN2021014).
Author information
Authors and Affiliations
Contributions
Yonghong Zhang and Jiasheng Zheng conceived and designed the protocol; Dandan Guo, Jianjun Li and Yinghua Zhang collected the data; Qi Wang and Dandan Guo wrote the manuscript; Qi Wang, Ning He, and Peng Zhao analyzed the data; Wenfeng Gao and Chunwang Yuan critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved by the Ethics Committee of Beijing You’An Hospital Affiliated to Capital Medical University [Jingyou Kelun Zi [2019]098]. This article is a retrospective study. Therefore, the Institutional waived the requirement to obtain distinct written informed consent from the patients.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Guo, D., Gao, W. et al. Individual surveillance by competing risk model for patients with hepatocellular carcinoma occurrence in all-cause cirrhosis. J Cancer Res Clin Oncol 149, 13403–13416 (2023). https://doi.org/10.1007/s00432-023-04911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04911-y