Abstract
Purpose
The aim of our study was to evaluate, under real-life conditions, survival of patients with advanced HCC (BCLC-C), either initially presenting in that stage or migrating from BCLC-A to BCLC-C within 2 years after curative LR/RFA, treated either with Atezolizumab–Bevacizumab or TKIs.
Methods
Sixty-four cirrhotic patients with advanced HCC, who either initially presented as BCLC-C and were treated with Atezo–Bev (group A, N = 23) or TKIs (group B, N = 15) or who migrated from BCLC-A to BCLC-C stage within 2 years after LR/RFA and were either treated with Atezo–Bev (group C, N = 12) or TKIs (group D, N = 14), were retrospectively evaluated.
Results
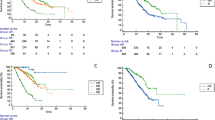
The four groups were comparable for all baseline parameters (demographics/platelets/liver disease etiology/diabetes/varices/Child–Pugh stage/ALBI grade) except for CPT score and MELD-Na. Using Cox-regression analysis, we observed that survival of group C after systemic treatment onset was significantly higher compared to group A (HR 3.71, 1.20–11.46, p = 0.02) and presented a trend to statistical significance when compared to group D (HR 3.14, 0.95–10.35, p = 0.06), adjusted for liver disease severity scores. When all BCLC-C patients classified as such due to PS only were excluded from the study, a trend for the same survival benefit in group C was shown, even in the most difficult-to-treat population with extrahepatic disease or macrovascular invasion.
Conclusion
Cirrhotic patients with advanced HCC initially diagnosed in BCLC-C, exhibit the worst survival irrespective of treatment schedule, whereas patients progressing to BCLC-C following disease recurrence after LR/RFA, seem to mostly benefit from Atezo–Bev, even patients with extrahepatic disease and/or macrovascular invasion. Liver disease severity seems to drive survival of these patients.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- BCLC:
-
Barcelona clinic liver cancer
- LR:
-
Liver resection
- RFA:
-
Radiofrequency ablation
- Atezo–Bev:
-
Atezolizumab–Bevacizumab
- TKI:
-
Tyrosine kinase inhibitor
- CPT:
-
Child–Pugh score
- MELD-Na:
-
Model for end-stage liver disease with sodium
- PDL1:
-
Programmed-death ligand 1
- VEGF:
-
Vascular endothelial growth factor
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- ORR:
-
Objective response rates
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- AFP:
-
A-fetoprotein
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- ALD:
-
Alcoholic liver disease
- NAFLD:
-
Non-alcoholic liver disease
- SAAG:
-
Serum ascites albumin gradient
- PS:
-
Performance status
- ECOG:
-
Eastern Cooperative Oncology Group
- NK cells:
-
Natural killer cells
References
Abou-Alfa GK, Meyer T, Cheng AL et al (2018) Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 379(1):54–63
Azam F, Latif MF, Farooq A et al (2019) Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol 12(3):728–736
Chen HY, Lu SN, Hung CH, Wang JH, Chen CH, Yen YH, Kuo YH, Kee KM (2020) Predicting outcomes for recurrent hepatocellular carcinoma within Milan criteria after complete radiofrequency ablation. PLoS One 15(11):e0242113
Cheng AL, Qin S, Ikeda M et al (2022) Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 76(4):862–873
Coates A, Porzsolt F, Osoba D (1997) Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer 33(7):1025–1030
De Re V, Rossetto A, Rosignoli A, Muraro E, Racanelli V, Tornesello ML, Zompicchiatti A, Uzzau A (2022) Hepatocellular carcinoma intrinsic cell death regulates immune response and prognosis. Front Oncol 12:897703. https://doi.org/10.3389/fonc.2022.897703
Dong SC, Bai DS, Wang FA et al (2022) Radiofrequency ablation is an inferior option to liver resection for solitary hepatocellular carcinoma ≤ 5 cm without cirrhosis: a population-based study with stratification by tumor size [published online ahead of print, 2022 Aug 8]. Hepatobiliary Pancreat Dis Int S1499-3872(22):00187-4.
Donne R, Lujambio A (2022) The liver cancer immune microenvironment: therapeutic implications for hepatocellular carcinoma [published online ahead of print, 2022 Aug 21]. Hepatology. https://doi.org/10.1002/hep.32740
Finn RS, Qin S, Ikeda M et al (2020) Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 382(20):1894–1905
Foerster F, Gairing SJ, Ilyas SI, Galle PR (2022) Emerging immunotherapy for HCC: a guide for hepatologists. Hepatology 75(6):1604–1626
Freitas ACT, Rampim AT, Nunes CP, Coelho JCU (2019) Impact of meld sodium on liver transplantation waiting list. Arq Bras Cir Dig 32(3):e1460. https://doi.org/10.1590/0102-672020190001e1460
Giraud J, Chalopin D, Blanc JF, Saleh M (2021) Hepatocellular carcinoma immune landscape and the potential of immunotherapies. Front Immunol 12:655697 (Published 2021 Mar 18)
Hack SP, Spahn J, Chen M et al (2020) IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation [published correction appears in Future Oncol. 2020 Oct;16(29):2371]. Future Oncol 16(15):975–989
Hasegawa K, Kokudo N, Makuuchi M et al (2013) Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey [published correction appears in J Hepatol. 2013 Sep;59(3):641]. J Hepatol 58(4):724–729
Johnson PJ, Berhane S, Kagebayashi C et al (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33(6):550–558
Kamath PS, Wiesner RH, Malinchoc M et al (2001) A model to predict survival in patients with end-stage liver disease. Hepatology 33(2):464–470
Kudo M (2021) Adjuvant immunotherapy after curative treatment for hepatocellular carcinoma. Liver Cancer. 10(5):399–403 (Published 2021 Sep 6)
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
Llovet JM, Brú C, Bruix J (1999a) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19(3):329–338
Llovet JM, Bustamante J, Castells A et al (1999b) Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 29(1):62–67
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM (2006) Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 243(2):229–235
Reig M, Forner A, Rimola J et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 76(3):681–693
Sas Z, Cendrowicz E, Weinhäuser I, Rygiel TP (2022) Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for new treatment options. Int J Mol Sci 23(7):3778
Sherman M (2008) Recurrence of hepatocellular carcinoma. N Engl J Med 359(19):2045–2047
Simonetti RG, Cammà C, Fiorello F, Politi F, D’Amico G, Pagliaro L (1991) Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci 36(7):962–972
Singh P, Toom S, Avula A, Kumar V, Rahma OE (2020) The immune modulation effect of locoregional therapies and its potential synergy with immunotherapy in hepatocellular carcinoma. J Hepatocell Carcinoma 10(7):11–17
Slovak R, Ludwig JM, Gettinger SN, Herbst RS, Kim HS (2017) Immuno-thermal ablations—boosting the anticancer immune response. J Immunother Cancer 5(1):78
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Yamashita T, Kudo M, Ikeda K et al (2020) REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 55(1):113–122
Yang W, Yan K, Goldberg SN, Ahmed M, Lee JC, Wu W, Zhang ZY, Wang S, Chen MH (2016) Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World J Gastroenterol 22(10):2993–3005
Yu JH, Lee JW (2020) Limitations of the Barcelona clinic liver cancer staging treatment strategy in hepatocellular carcinoma patients with performance status 1. Ann Transl Med 8(17):1042
Zerbini A, Pilli M, Fagnoni F, Pelosi G, Pizzi MG, Schivazappa S, Laccabue D, Cavallo C, Schianchi C, Ferrari C, Missale G (2008) Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother 31:271–282
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: PS, SA, EI; methodology: PS, SA, MD, EI; software: GP; validation: BG, SI, EI; formal analysis: GP; investigation: PS; resources: PS, SA, MD, SI, BG, PN; data curation: PS, GP, EI; writing—original draft preparation: PS, EI; writing—review and editing: PS, EI; visualization: PS, GP; supervision: EI; project administration: PS, SA, MD, BG, SI, PN, GP, EI; funding acquisition: none.
Corresponding author
Ethics declarations
Conflict of interest
All authors of this article have no conflict of interest to declare.
Ethics approval
Written informed consent was collected from all the alive patients and the study protocol was in accordance with the Declaration of Helsinki and was evaluated and approved by the Ethics Committee/Scientific Board of the General and Oncology Hospital of Kifisia ‘‘Agioi Anargyroi”, Athens, Greece (Date 18-07-2022, No. 987).
Informed consent
Written informed consent was collected from all the alive patients included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spyridon, P., Antonia, S., Dionysia, M. et al. Efficacy of Αtezolizumab–Βevacizumab in BCLC-C cirrhotic patients with hepatocellular carcinoma according to the type of disease progression, the type of BCLC-C and liver disease severity. J Cancer Res Clin Oncol 149, 9253–9261 (2023). https://doi.org/10.1007/s00432-023-04846-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04846-4