Abstract
Purpose
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm characterized by recurrent genetic aberration in leukemic stem cells, namely Philadelphia chromosome caused by reciprocal translocation t(9;22)(q34;q11). In our study, we analyzed the telomeric complex expression and function in the molecular pathogenesis of CML.
Methods
We employed CD34+ primary leukemic cells, comprising both leukemic stem and progenitor cell populations, isolated from peripheral blood or bone marrow of CML patients in chronic and blastic phase to analyze the telomere length and telomeric-associated proteins.
Results
The reduction in telomere length during disease progression was correlated with increased expression of BCR::ABL1 transcript and the dynamic changes were neither associated with the enzymatic activity of telomerase nor with gene copy number and expression of telomerase subunits. Increased expression of BCR::ABL1 was positively correlated with expression of TRF2, RAP1, TPP1, DKC1, TNKS1, and TNKS2 genes.
Conclusions
The dynamics of telomere length changes in CD34+ CML cells is dependent on the expression level of BCR::ABL, which promotes the expression of certain shelterins including RAP1 and TRF2, as well as TNKS, and TNKS2, and results in telomere shortening regardless of telomerase activity. Our results may allow better understanding of the mechanisms responsible for the genomic instability of leukemic cells and CML progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myeloid leukemia (CML) is a model neoplastic disease and constitutes an excellent example of translating basic knowledge into targeted therapy and clinical benefit (Jabbour and Kantarjian 2022). CML is characterized by recurrent genetic aberration in leukemic stem cells, namely the Philadelphia chromosome caused by reciprocal translocation t(9;22)(q34;q11) that leads to the formation of BCR::ABL1 fusion oncogene (Nowell and Hungerford 1960; Rowley 1973). The hybrid gene BCR::ABL1 undergoes translation into chimeric protein, which exerts constitutive tyrosine kinase activity and phosphorylates target proteins to facilitate survival and expansion of leukemic stem cells (Lin-CD34 + CD38 −) and progenitor cells (CD34 + CD38 +). Thus, CML is frequently described as leukemia stem cell (LSCs)-derived, but leukemia progenitor cell (LPCs)-driven disease (Marley and Gordon 2005). Progression of CML is characterized by successive increase in amount of blast cells in blood and bone marrow and is classified by phases: chronic phase (CML-CP), accelerated phase (CML-AP), and advanced blastic phase (CML-BP) also called blast crisis (Jabbour and Kantarjian 2022). A drug resistance and/or disease progression in CML despite all major milestones constitutes a significant clinical problem in a number of patients (Perrotti et al. 2010; Skorski et al. 2012). Searching for new markers useful as potential prognostic and/or predictive factors in CML is still a challenge and the subject of many studies (Wenn et al. 2015; Niederwieser and Kröger 2022).
One of such potential prognostic markers postulated were changes in telomere length because it was shown that telomere length was shorter in CML cells as compared to age-matched healthy individuals or BCR::ABL1-negative T lymphocytes from the same patients (Brümmendorf et al. 2000). Furthermore, telomere shortening was accelerated as the disease progressed from CML-CP to CML-BP, with a shortening rate approximately 10× higher than in normal controls (Iwama et al. 1997; Drummond et al. 2004; Wang et al. 2014; Bouillon et al. 2018). Unraveling the mechanisms and the role of telomeric complex in BCR::ABL1-mediated genomic instability may contribute to the development of new strategies for preventing or counteracting resistance phenotype and malignant progression of the disease. This may create new and unique therapeutic opportunities, as shown in acute myeloid leukemia as an effective strategy to eradicate leukemia stem cells (Bruedigam et al. 2014; Mascarenhas et al. 2021).
Telomere maintenance in malignant cells is associated with reactivation of telomerase, and/or the telomerase-independent alternative lengthening of telomeres (ALT) (Gao and Pickett 2022). It is well known that telomere maintenance is regulated not only by telomerase, but also by Telomeric Repeat-containing RNA (TERRA) (Silva et al. 2021) and various telomere-associated proteins, such as the shelterin complex composed of 6 proteins: telomeric repeat-binding factors 1 and 2 (TRF1 and TRF2), protection of telomeres (POT1), TRF2-interacting protein 1 (RAP1), TRF1-interacting nuclear factor 2 (TINF2), and TIN2-interacting protein 1 (TPP1), as well as other telomeric-associated proteins (telomerase associated protein (TEP1) and tankyrase). TRF1 and TRF2 along with TINF2, which prevents TRF1/TRF2 degradation by tankyrase, form the central hub of the shelterin complex that protects telomeres from being recognized as DNA double-strand breaks thereby avoiding inappropriate end-joining and DNA repair. Thus, TRF1 and TRF2, and PinX1 (TRF-interacting telomerase inhibitor 1) act as negative regulators of telomere length, while telomerase and tankyrase are positive regulators (Hockemeyer and Collins 2015; de Lange 2018). Telomere length in normal and malignant cells is regulated by a delicate balance between these factors (Augereau et al. 2011). Interestingly, so far most studies on CML cells focused on the analysis of the dependence of telomere length on telomerase activity (Brümmendorf et al. 2000; Drummond et al. 2004, 2005; Wenn et al. 2015; Bouillon et al. 2018), but characterization of the telomeric complex and telomere maintenance, including shelterin complex, in CD34+ CML cells has never been analyzed in detail.
In this work, we have investigated the effects of BCR::ABL1-mediated changes on expression of shelterin complex, TERT, telomerase RNA component (TERC) and dyskerin pseudouridine synthase 1 (DKC1) in CML cells including CD34+ primary cells isolated from peripheral blood or bone marrow of CML patients at different stages of disease. We showed for the first time that BCR::ABL1 promoted telomere shortening independent of telomerase activity by overexpression of some shelterin genes.
Materials and methods
Patient samples and cell lines
Blood or bone marrow samples were obtained from 76 CML patients (55 in CML-CP and 21 in CML-BP) with confirmed Philadelphia chromosome and BCR::ABL1 translocation and from 4 healthy volunteers. A peripheral blood mononuclear cells were isolated from CML patients or healthy blood donors using Histopaque 1077 and Histopaque 1119 (Sigma-Aldrich, Saint Louis, MO, USA), then progenitor CML CD34 + cells were selected using magnetic beads system EasySep CD34 + Positive Selection Kit (StemCell Technologies, Vancouver, Canada) according to the manufacturer’s recommendation. CD34 + cells were maintained in Iscove’s modified Dulbecco’s medium (IMDM; Lonza, Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS) and growth factors: 2 ng/ml hrIL-3, 10 ng/ml hrGM-CSF and 2 ng/ml hrSCF (PeproTech, Cranbury, NJ, USA) at 37 °C in a humidified atmosphere of 5% CO2.
32D clone 3, interleukin (IL)-3-dependent cell line, and their BCR::ABL1-transformed counterparts were described before (Koptyra et al. 2006). The cells were cultured in Roswell Park Memorial Institute 1640 medium (RPMI, Lonza, Basel, Switzerland) with 10% FBS and 4 ng/ml mrIL-3 (PeproTech, Cranbury, NJ, USA) at 37 °C in a humidified atmosphere of 5% CO2.
RNA isolation and cDNA synthesis
Total RNA was extracted from peripheral blood samples using QIAamp RNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Reverse transcription was done with Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland) following the manufacturer's protocol.
Real-time qPCR analysis
The levels of the mRNA expression of BCR::ABL1, TRF1, TRF2, RAP1, POT1, TINF2, TPP1, TNKS1, TNKS2, DKC1, TERC, and TERT were measured using LightCycler® 480 Probes Master and Universal Probe Library (UPL) (Roche, Basel, Switzerland). The reactions were performed using LightCycler® 480 instrument (Roche, Basel, Switzerland) in a final volume of 10 µl. B2M and GUSB were used as reference genes (Supplementary Table 1).
Fluorescence in situ hybridization (FISH)
FISH was performed using probes for: BCR/ABL1 t(9;22) fusion (KBI-10005, Kreatech, Amsterdam, Netherlands), TERT (5p15) (KBI-40113, Kreatech, Amsterdam, Netherlands), or TERC (3q26)/3q11 (KBI-10110, Kreatech, Amsterdam, Netherlands). For FISH experiments, the procedure used has been described elsewhere (Deregowska et al. 2020).
Telomere length analysis and telomerase activity
Average telomere length was analyzed by Southern blotting analysis and at the level of the single cell by FISH. The genomic DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) and the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) for patients’ samples and murine cells, respectively, according to the manufacturer’s protocols. Terminal restriction fragments (TRF) length was measured by Southern blot method, using TeloTAGGG telomere length assay kit (Roche, Basel, Switzerland) following the manufacturer's protocol as described previously (Wnuk et al. 2014). In single cells, metaphase chromosomes were prepared as described elsewhere (Deregowska et al. 2020), and then Q-FISH was performed with the Telomere PNA FISH kit/Cy3 (Dako, Glostrup, Denmark) according to the protocol provided by the manufacturer’s recommendation.
Telomerase activity (TA) was measured with a TeloTAGGG Telomerase PCR ELISA kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed in GraphPad Prism 6.07 software (GraphPad Software, Inc., La Jolla, CA, USA) by t test with the Mann–Whitney test and by one-way ANOVA and with the Tukey’s multiple comparison test. The correlation analysis was performed using linear correlation (Pearson r) test.
Results
Decreased telomere length correlates with increased BCR::ABL1 gene expression in CML cells
With the aim of assessing the prognostic importance of changes in the length of telomeres in leukemic leukocytes of patients with CML and discovering the mechanisms responsible for the dynamism of changes in telomere length, we have conducted research on leukocytes including CD34+ primary leukemic cells, comprising both leukemic stem and progenitor populations isolated from patients at the CML-CP and CML-BP stages of the disease. The cells of all patients with the presence of the BCR::ABL1 gene (Fig. 1a) were characterized by BCR::ABL1 transcriptional activity (Fig. 1b). Comparative analysis of the expression of the fusion gene BCR::ABL1 by qPCR within the group showed a 2.5 fold increase in BCR::ABL1 expression in the cells of CML-BP patients in relation to CML-CP patients. However, it should be noted that a larger variability in the expression profile of BCR::ABL1, in general, was observed in the CML-BP group rather than in cells isolated from CML-CP patients.
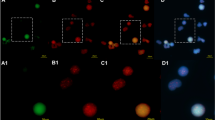
Changes in telomere length depending on CML phase and BCR::ABL1 expression level in CD34+ primary leukemic cells isolated from patients at different stages of disease (CML-CP and CML-BP). a Interphase and metaphase FISH images with typical BCR::ABL1 gene rearrangements observed among the study groups using LSI BCR/ABL1 probe localized on chr.22q11.2 and chr.9q34, respectively (signal pattern 2F1R1G). Sequences flanking the ABL1 (9q34) gene are direct labeled with PlatinumBright™550. Sequences flanking the BCR (22q11) gene are direct-labeled PlatinumBright™495. b The BCR::ABL1 expression level normalized to B2M and GUSB measured with qPCR (non-parametric Student’s t test and Mann–Whitney post-test) ***p < 0.001, n = 71. c Representative images from Southern blot analysis. Mean TRF length (kbp) is shown in the legend of the particular lanes. Lanes: 1 and 15 DIG-molecular weight marker (MWM), [0.8–21.2], 2 and 15 control DNA [7.6 ± 0.2] (PC). d Comparison of telomere length measured by TRF method in leukocytes isolated from healthy donors (N) and patients at different stages of disease: CML-CP, CML-BP expressed as kbp, (ANOVA and Tukey’s a posteriori test). ***p < 0.001, *p < 0.05, n = 47. e Correlation between the level of mRNA BCR::ABL1 expression and telomere length measured by TRF method (Pearson’s (r) p < 0.05). f Representative microphotographs of Q-FISH for CML CD34 + primary cells isolated from CML-CP, CML-BP patients. Telomere PNA FISH kit/Cy3 (Dako) was used for telomere labeling. Nuclei were counterstained by DAPI. g Means of telomere area (pixel per spot) per CML CD34 + primary cell isolated from CML-CP, CML-BP patients measured by Q-FISH with PNA technique, (non-parametric student t test and Mann–Whitney post-test). **p < 0.01, n = 30. h Correlation between level of mRNA BCR::ABL1 expression and telomere length measured by Q-FISH method (Pearson’s (r) p < 0.05). i Correlation between age and telomere length. (Pearson’s (r) p < 0.05), n = 43. Red dots—CML-BP cells, black dots—CML-CP cells. j Southern blot analysis of telomere length in murine myeloid 32D clone 3: parental and transfected with the BCR::ABL1 gene, MWM—molecular weight marker, PC—positive control. Densitometric profile was performed to correspond to bands of DNA marker using ImageJ with gel analysis module
We decided to verify the model of telomere biology in CML proposed by Brümmendorf et al. on the basis of two techniques, TRF method and Q-FISH analysis, and correlation analysis between obtained results and the level of expression of the BCR::ABL1 gene (Brümmendorf et al. 2000). Additionally, we attempted to explain the phenomenon of changes in telomere length in patients with CML, as observed by Brümmendorf and other researchers. TRF and Q-FISH analysis confirmed that the mean length of telomeres in the CML-BP phase was significantly shorter in comparison with CML-CP phase telomeres (Fig. 1c–g). Moreover, the correlation analysis showed that the dynamics of this process may be related to the level of BCR::ABL1 expression, that is, cells with higher BCR::ABL1 expression had significantly shorter telomeres (Fig. 1e, h). The analysis of the relationship between age of CML patients and mean length of telomeres has shown that telomere length slightly decreased with age (Fig. 1i).
To investigate the effect of BCR::ABL1 tyrosine kinase on telomere length, 32D cells expressing BCR::ABL1, and the parental cell line 32D clone 3 not expressing BCR::ABL1 were compared (Fig. 1j). Telomere lengths were quantified using Southern blot, and the results showed heterogenization of telomere length in BCR::ABL1-expressing and non-expressing cells.
Changes in the levels of TERT and DKC1 expression do not affect a global telomerase activity in CML cells
Analysis of the number of copies of TERT and TERC genes by FISH did not show statistically relevant differences between CML-BP CD34+ and CML-CP CD34+ cells. There was an average of 2 for TERC and TERT genes per cell (Supplementary Fig. 1S). Only in a few clinical cases, we observed that the average number of copies of the TERT gene was over 2 (in three CML-CP patients and in one CML-BP patient). Comparative analysis of expression profiles of TERC and TERT in two groups of patients showed a statistically significant increase only in the expression of TERT in the CML-BP group of patients (Fig. 2a–b). However, it should be noted that the level of TERT gene expression was at a very low level, undetectable in many samples, with a tendency to increase in CML-BP.
BCR::ABL1-mediated effect on TERC, TERT, and DKC1 expression of telomerase components during CML progression. a–c The TERC (n = 54), TERT (n = 33), and DKC1 (n = 42) expression levels normalized to B2M and GUSB measured with qPCR **p < 0.01(non-parametric Student’s t test and Mann–Whitney post-test). d PCR ELISA measurement of telomerase activity, n = 10 (non-parametric student t test and Mann–Whitney post-test). e–g Correlation between BCR::ABL1 and TERC, TERT or DKC1 expression level, respectively (Pearson’s (r)) p < 0.05
Next, we checked the transcriptional activity of the DKC1 gene—nucleolar protein, which is responsible for maintaining TERC stability by interacting with H/ACA consensus sequence in TERC. The conducted comparison between the expression of the DKC1 gene in two groups of CML CD34+ showed a statistically significant increase (2.1-fold) of DKC1 expression in CML-BP cells in comparison with CML-CP cells (Fig. 2c). Additional correlation analysis conducted on CML CD34+ cells showed a statistically significant correlation between DKC1 expression and the BCR::ABL1 gene (r = 0.68 p < 0.0001) (Fig. 2g), and, interestingly, such significance was not found for TERC or TERT genes (Fig. 2e, f). Moreover, there was no significant difference in telomerase activity between CML-CP and CML-BC cells (Fig. 2d).
The shelterin gene expression correlates with the level of BCR::ABL1 in CML cells
Comparative analysis of the expression profile of genes of the telomere complex, such as TRF1, TRF2, POT1, RAP1, TTP1, TNKS1, and TNKS2 in CML-CP and CML-BP cells, showed a statistically significant increase in the expression of two genes: TNKS1 and RAP1 in CML-BP cells (p < 0.01) (Fig. 3a). The average level of RAP1 expression was 1.4-fold higher for CML-BP cells and 1.72-fold higher for TKNS1 in relation to CML-CP cells, respectively. A comparison between the expression of different genes of the shelterin complex and the level of BCR::ABL1 transcription showed statistically significant correlations with the following genes: RAP1 (p = 0.0006), TRF2 (p = 0.003), TPP1 (p = 0.01), TNKS1 (p < 0.0001) and TNKS2 (p = 0.017) (Fig. 3b).
CML phase and BCR::ABL1 expression-mediated changes in the expression of TRF1 (n = 52), TRF2 (n = 53), POT1 (n = 52), RAP1 (n = 48), TINF2 (n = 49), TPP1 (n = 49), TNKS1 (n = 51) and TNKS2 (n = 49) genes. a Comparison of RAP1, POT1, TRF1, TRF2, TPP1, TNKS1 and TNKS2 gene expression between CML-CP and CML-BP patients. The gene expression was normalized to B2M and GUSB and measured with qPCR, **p < 0.01 (non-parametric student t test and Mann–Whitney post-test). b Correlation between BCR::ABL1 and TRF1, TRF2, POT, RAP1, TINF2, TPP1, TNKS1 or TNKS2 expression levels (Pearson’s (r), p < 0.05)
Discussion
During CML progression, the loss of genetic stability is manifested not only by a general increase in point mutations, DNA breakages, oxidative DNA damage, or disturbances of the epigenome, but also by changes in telomeric sequences (Boultwood et al. 1999; de Oliveira et al. 2022). The analysis of telomere length in hematopoietic (HSCs) and LSCs from the same patient shows that the average length of telomere in LSCs is much shorter, and this shortening correlates with the leukemic clone size (Bouillon et al. 2018). Moreover, according to the Hasford score, high-risk patients at diagnosis, reveal significantly greater telomere shortening rate compared to low-risk score patients, while intermediate-risk score patients exhibit an intermediate telomere shortening rate (Drummond et al. 2004). The obtained results confirm that telomeres were significantly shorter in CML-BP cells compared to cells from patients in CML-CP (Drummond et al. 2004). Thus, telomere shortening can be considered as a novel prognosis marker complementary to already established markers (Wang et al. 2014). The correlation analysis of the telomere length and the level of BCR::ABL1 expression suggests that BCR::ABL1 may induce dynamic changes in telomere length. Moreover, telomere shortening may contribute to senescence-associated inflammation and in turn disease progression in CML (Braig et al. 2014). CML cells might present nonrandom individual telomere length changes, such as shortening with different shorting rates and lengthening of telomeres located at some specific chromosome ends (Samassekou et al. 2009). It has been shown that the dynamics of individual telomere lengths might lead to telomere position effects, and in consequence inappropriate gene expression at subtelomeric regions (Koering et al. 2002). The analysis of the relationship between age of CML patients and mean length of telomeres has shown that telomere length slightly decreased with age. However, generally telomere length in CML-BP cells was still shorter than in CML-CP cells, so the age-related changes in telomere length during CML do not seem to be the major factor responsible for telomere shortening.
In cancer cells, the lengthening of telomere sequences takes place through the reactivation of telomerase enzyme activity or alternatively through the process of recombination (Okamoto and Seimiya 2019). The decreasing effectiveness of these mechanisms leads to excessive shortening of telomeres, resulting in chromosome instability, which often leads to cellular heterogeneity related to defective mechanisms of apoptosis (Murnane et al. 2012). Furthermore, chromosome changes can promote the production of many factors that work mainly locally, for instance the cytokines or growth factors, which may lead to inflammation or tumor growth (Andriani et al. 2016). Thus, understanding the mechanisms that regulate the length of telomeres may shed light on the processes of cell selection and adaptation that occur during the development of cancer, including CML. For these purposes, it would be interesting to find out the molecular factors that control the activity of telomerase. Telomerase, as a ribonucleoprotein enzyme complex, is composed of a subunit of reverse transcriptase (TERT) and an RNA component (TERC). The activity of human telomerase is controlled on three levels, namely, on the level of transcription, the assembly of subunits into an active enzyme, as well as of direct interaction of telomerase with proteins from the telomere complex.
Our comprehensive analysis of the activity of telomerase in CML CD34+ cells does not confirm earlier observations, which pointed to changes in the activity of this enzyme depending on the phase of disease in leukocyte cells of patients with CML (Ohyashiki et al. 1997). However, it should be noted that in the aforementioned work, the authors analyzed unfractionated cells from 33 CML-CP patients and 21 CML-BP patients. The cells at CML-BP exhibited a significant increase in telomerase activity (TA) (p = 0.016) and, at the same time, a statistically significant decrease in telomere length from 6.13 ± 1.68 kb in CML-CP to 4.53 ± 0.72 kb in CML-BP at p = 0.0005). The authors did not correlate their results with the level of expression or activity of BCR::ABL1 kinase. Drummond et al. arrived at similar conclusions, showing a lack of overexpression of TERT and lowered levels of TERC expression in CD34 + cells of CML-CP patients as compared with healthy subjects (Drummond et al. 2005). They postulated that the observed increase in TA in peripheral blood cells of patients with CML may be related to a heightened proportion of cells released from bone marrow into the periphery, rather than a true increase in intracellular telomerase activity.
Nevertheless, the association of telomerase upregulation with CML progression has been reported (Keller et al. 2009). Based on these results, telomere length, at least in the context of intact cell cycle checkpoints, could represent a valuable prognostic and/or predictive biomarker for disease progression, response to TKIs, and potentially for maintenance of response upon cessation of TKI treatment.
The analysis of expression profiles of TERC and TERT in two groups of patients showed a statistically significant increase only in the expression of TERT in the CML-BP group of patients. Thereby, the obtained results do not confirm earlier observations, which point to lowered expression of TERT along with the progression of CML (Campbell et al. 2006). Campbell et al., comparing the gene expression in the CML CD34+ cells isolated from 22 CML patients’ samples to the normal CD34+ cells, showed that expression of TERT was downregulated in over half of the samples from patients in the chronic phase, significantly downregulated in two out of three patients in the accelerated phase and in all CML CD34+ cells isolated from patients in blastic phase. The same authors also postulated that lowered transcription of TERT in the CML-BP stage is associated with the levels of C-MYC, the expression of which decreased as the disease progresses. Due to these divergences, extended research in this area is required. Nevertheless, our results show that the level of expression and number of copies of TERT cannot be considered as the main cause of changes in telomere length during progression. In this context, we checked the transcriptional activity of the DKC1 gene–nucleolar protein, which is responsible for maintaining TERC stability. The role of DKC1 in the progression and development of hematopoietic and solid tumors has been already described i.e., DKC1 dysfunction leads to diminished TERC levels, a decrease in telomerase activity, and premature telomere shortening in males (Montanaro et al. 2010; Hirvonen et al. 2019). The conducted comparison between the expression of the DKC1 gene in two groups of CML CD34+ showed a significant increase (2.1-fold) of DKC1 expression in CML-BP cells in comparison with CML-CP cells. This result is in contrast with the observed decrease in the length of telomeres in CML-BP. This may suggest that DKC1 overexpression in CML cells is not related to telomerase activity. A likely explanation for this biological phenomenon is the increase of CML cancer cells’ demand for DKC1 due to its role in post-transcriptional modification of rRNA necessary for the maintenance of an effective process of translation (Ge et al. 2010; Jack et al. 2011).
Comparative analysis of the expression profile of genes of the telomere complex showed a significant increase in the expression of two genes: TNKS1 and RAP1 in CML-BP cells. Campbell et al. 2006 previously showed that the expression of telomeric-associated proteins TEP1, TRF1, TRF2, TNKS1, and PinX1 was elevated in the majority of CML-CP and CML-AP patients and decreased during disease progression, with the exception of TEP1 (Campbell et al. 2006). However, it ought to be noted that the analysis of the expression of the genes studied had not been correlated with the expression of BCR::ABL1 in individual samples, and the analysis was performed on one reference gene (B2M), which may have an impact on the obtained results, while our results were normalized to B2M and GUSB.
Moreover, contrary to the other researchers we have shown a positive correlation between increased expression of TRF2, RAP1, TTP1, TNKS1, and TNKS2 genes and the level of expression of BCR::ABL1, and also simultaneously with a decrease in the length of telomeres. Nevertheless, one ought to remember that an increase in the expression of RAP1 observed here may not be related to changes in telomeres and could be merely another form of adaptation of CML cells to increased metabolic activity characteristic for cancer cells (Deregowska and Wnuk 2021). It is well known that RAP1 is a pleiotropic protein that is responsible for the regulation of cell metabolism, the production of conditions associated with inflammation, response to oxidative stress (Cai et al. 2017) and regulation of hematopoietic stem cell survival (Khattar et al. 2019). Therefore, due to the observed correlation between increased expression of BCR::ABL1 and levels of TRF2 expression, an alternative explanation may also be found in the following scenario: the increase of expression of BCR::ABL1 during the progression of CML leads to an increase in levels of shelterin complex proteins, including the overexpression of TRF1 and TRF2, which are known to be negative regulators of telomere length, and whose binding to telomeres is dependent on posttranslational modification of the poly-ADP-ribosylate by tankyrases 1 and 2 (van Steensel et al. 1998; Smogorzewska et al. 2000; Smogorzewska and de Lange 2004). Furthermore, overexpression of TRF1 and TRF2 may promote the nucleolytic activity of XPF on chromosome endings, leading to acceleration of telomere shortening (Muñoz et al. 2005).
Conclusion
In summary, we show that the telomere length dynamics in CML cells including CD34+ cells is strictly related to the level of expression of BCR::ABL1, which stimulates an increase in expression of some shelterin genes, including TRF2, RAP1 and TPP1, as well as other telomere-associated proteins: DKC1, TNKS1, and TNKS2, promoting the shortening of telomeres regardless of telomerase activity. Our results may help in better understanding of the mechanisms responsible for the loss of genome stability in CD34+ cells and CML progression. We believe that our results may also help in the future to find new therapeutic targets in leukemic stem cells and the development of effective therapy especially for advanced phases of the disease, but also may be helpful in other hematological malignancies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Andriani GA, Almeida VP, Faggioli F et al (2016) Whole chromosome instability induces senescence and promotes SASP. Sci Rep 6:35218. https://doi.org/10.1038/srep35218
Augereau A, de T’kint RC, Simonet T et al (2011) Telomeric damage in early stage of chronic lymphocytic leukemia correlates with shelterin dysregulation. Blood 118:1316–1322. https://doi.org/10.1182/blood-2010-07-295774
Bouillon A-S, Ventura Ferreira MS, Awad SA et al (2018) Telomere shortening correlates with leukemic stem cell burden at diagnosis of chronic myeloid leukemia. Blood Adv 2:1572–1579. https://doi.org/10.1182/bloodadvances.2018017772
Boultwood J, Fidler C, Shepherd P et al (1999) Telomere length shortening is associated with disease evolution in chronic myelogenous leukemia. Am J Hematol 61:5–9. https://doi.org/10.1002/(sici)1096-8652(199905)61:1%3c5::aid-ajh2%3e3.0.co;2-4
Braig M, Pällmann N, Preukschas M et al (2014) A ‘telomere-associated secretory phenotype’ cooperates with BCR-ABL to drive malignant proliferation of leukemic cells. Leukemia 28:2028–2039. https://doi.org/10.1038/leu.2014.95
Bruedigam C, Bagger FO, Heidel FH et al (2014) Telomerase inhibition effectively targets mouse and human AML stem cells and delays relapse following chemotherapy. Cell Stem Cell 15:775–790. https://doi.org/10.1016/j.stem.2014.11.010
Brümmendorf TH, Holyoake TL, Rufer N et al (2000) Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood 95:1883–1890
Cai Y, Kandula V, Kosuru R et al (2017) Decoding telomere protein Rap 1: Its telomeric and nontelomeric functions and potential implications in diabetic cardiomyopathy. Cell Cycle 16:1765–1773. https://doi.org/10.1080/15384101.2017.1371886
Campbell LJ, Fidler C, Eagleton H et al (2006) hTERT, the catalytic component of telomerase, is downregulated in the haematopoietic stem cells of patients with chronic myeloid leukaemia. Leukemia 20:671–679. https://doi.org/10.1038/sj.leu.2404141
de Lange T (2018) Shelterin-mediated telomere protection. Annu Rev Genet 52:223–247. https://doi.org/10.1146/annurev-genet-032918-021921
de Oliveira FM, Jamur VR, Merfort LW et al (2022) Three-dimensional nuclear telomere architecture and differential expression of aurora kinase genes in chronic myeloid leukemia to measure cell transformation. BMC Cancer 22:1024. https://doi.org/10.1186/s12885-022-10094-5
Deregowska A, Wnuk M (2021) RAP1/TERF2IP—a multifunctional player in cancer development. Cancers 13:5970. https://doi.org/10.3390/cancers13235970
Deregowska A, Pepek M, Pruszczyk K et al (2020) Differential regulation of telomeric complex by BCR-ABL1 kinase in human cellular models of chronic myeloid leukemia-from single cell analysis to next-generation sequencing. Genes (basel). https://doi.org/10.3390/genes11101145
Drummond M, Lennard A, Brûmmendorf T, Holyoake T (2004) Telomere shortening correlates with prognostic score at diagnosis and proceeds rapidly during progression of chronic myeloid leukemia. Leuk Lymphoma 45:1775–1781. https://doi.org/10.1080/10428190410001693542
Drummond MW, Hoare SF, Monaghan A et al (2005) Dysregulated expression of the major telomerase components in leukaemic stem cells. Leukemia 19:381–389. https://doi.org/10.1038/sj.leu.2403616
Gao J, Pickett HA (2022) Targeting telomeres: advances in telomere maintenance mechanism-specific cancer therapies. Nat Rev Cancer 22:515–532. https://doi.org/10.1038/s41568-022-00490-1
Ge J, Rudnick DA, He J et al (2010) Dyskerin ablation in mouse liver inhibits rRNA processing and cell division. Mol Cell Biol 30:413–422. https://doi.org/10.1128/MCB.01128-09
Hirvonen EAM, Peuhkuri S, Norberg A et al (2019) Characterization of an X-chromosome-linked telomere biology disorder in females with DKC1 mutation. Leukemia 33:275–278. https://doi.org/10.1038/s41375-018-0243-5
Hockemeyer D, Collins K (2015) Control of telomerase action at human telomeres. Nat Struct Mol Biol 22:848–852. https://doi.org/10.1038/nsmb.3083
Iwama H, Ohyashiki K, Ohyashiki JH et al (1997) The relationship between telomere length and therapy-associated cytogenetic responses in patients with chronic myeloid leukemia. Cancer 79:1552–1560. https://doi.org/10.1002/(SICI)1097-0142(19970415)79:8%3c1552::AID-CNCR17%3e3.0.CO;2-X
Jabbour E, Kantarjian H (2022) Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring. American J Hematol 97:1236–1256. https://doi.org/10.1002/ajh.26642
Jack K, Bellodi C, Landry DM et al (2011) rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell 44:660–666. https://doi.org/10.1016/j.molcel.2011.09.017
Keller G, Brassat U, Braig M et al (2009) Telomeres and telomerase in chronic myeloid leukaemia: impact for pathogenesis, disease progression and targeted therapy. Hematol Oncol 27:123–129. https://doi.org/10.1002/hon.901
Khattar E, Maung KZY, Chew CL et al (2019) Rap1 regulates hematopoietic stem cell survival and affects oncogenesis and response to chemotherapy. Nat Commun 10:5349. https://doi.org/10.1038/s41467-019-13082-9
Koering CE, Pollice A, Zibella MP et al (2002) Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep 3:1055–1061. https://doi.org/10.1093/embo-reports/kvf215
Koptyra M, Falinski R, Nowicki MO et al (2006) BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood 108:319–327. https://doi.org/10.1182/blood-2005-07-2815
Marley SB, Gordon MY (2005) Chronic myeloid leukaemia: stem cell derived but progenitor cell driven. Clin Sci 109:13–25. https://doi.org/10.1042/CS20040336
Mascarenhas J, Komrokji RS, Palandri F et al (2021) Randomized, single-blind, multicenter phase II study of two doses of imetelstat in relapsed or refractory myelofibrosis. JCO 39:2881–2892. https://doi.org/10.1200/JCO.20.02864
Montanaro L (2010) Dyskerin and cancer: more than telomerase. The defect in mRNA translation helps in explaining how a proliferative defect leads to cancer. J Pathol 222:345–349. https://doi.org/10.1002/path.2777
Muñoz P, Blanco R, Flores JM, Blasco MA (2005) XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat Genet 37:1063–1071. https://doi.org/10.1038/ng1633
Murnane JP (2012) Telomere dysfunction and chromosome instability. Mutat Res 730:28–36. https://doi.org/10.1016/j.mrfmmm.2011.04.008
Niederwieser C, Kröger N (2022) Transplantation in CML in the TKI era: who, when, and how? Hematology 2022:114–122. https://doi.org/10.1182/hematology.2022000329
Nowell PC, Hungerford DA (1960) A minute chromosome in human granulocytic leukemia. Science 132:1497–1498
Ohyashiki K, Ohyashiki JH, Iwama H et al (1997) Telomerase activity and cytogenetic changes in chronic myeloid leukemia with disease progression. Leukemia 11:190–194
Okamoto K, Seimiya H (2019) Revisiting telomere shortening in cancer. Cells. https://doi.org/10.3390/cells8020107
Perrotti D, Jamieson C, Goldman J, Skorski T (2010) Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Investig 120:2254–2264. https://doi.org/10.1172/JCI41246
Rowley JD (1973) A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and giemsa staining. Nature 243:290–293. https://doi.org/10.1038/243290a0
Samassekou O, Ntwari A, Hébert J, Yan J (2009) Individual telomere lengths in chronic myeloid leukemia. Neoplasia 11:1146-IN6. https://doi.org/10.1593/neo.09836
Silva B, Arora R, Bione S, Azzalin CM (2021) TERRA transcription destabilizes telomere integrity to initiate break-induced replication in human ALT cells. Nat Commun 12:3760. https://doi.org/10.1038/s41467-021-24097-6
Skorski T (2012) Genetic mechanisms of chronic myeloid leukemia blastic transformation. Curr Hematol Malig Rep 7:87–93. https://doi.org/10.1007/s11899-012-0114-5
Smogorzewska A, de Lange T (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73:177–208. https://doi.org/10.1146/annurev.biochem.73.071403.160049
Smogorzewska A, van Steensel B, Bianchi A et al (2000) Control of human telomere length by TRF1 and TRF2. Mol Cell Biol 20:1659–1668. https://doi.org/10.1128/mcb.20.5.1659-1668.2000
van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92:401–413. https://doi.org/10.1016/S0092-8674(00)80932-0
Wang L, Xiao H, Zhang X et al (2014) The role of telomeres and telomerase in hematologic malignancies and hematopoietic stem cell transplantation. J Hematol Oncol. https://doi.org/10.1186/s13045-014-0061-9
Wenn K, Tomala L, Wilop S et al (2015) Telomere length at diagnosis of chronic phase chronic myeloid leukemia (CML-CP) identifies a subgroup with favourable prognostic parameters and molecular response according to the ELN criteria after 12 months of treatment with nilotinib. Leukemia 29:2402–2404. https://doi.org/10.1038/leu.2015.245
Wnuk M, Lewinska A, Gurgul A et al (2014) Changes in DNA methylation patterns and repetitive sequences in blood lymphocytes of aged horses. Age 36:31–48. https://doi.org/10.1007/s11357-013-9541-z
Funding
This work was supported by the OPUS grant from the Polish National Science Center (UMO-2015/19/B/NZ5/03501) (TS).
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision: TS and MW; methodology: AD, MP, IS, MMM and KP; provision of samples and patients formal analysis: IS, MD, JN-K, IS, WS and TS; data analysis and interpretation: AD, MP, IS, MMM, KP, MW, and TS; original draft preparation: AD, MP, MW, and TS; visualizations: AD, MW; resources, TS and MW; funding acquisition: TS. All authors have read and agreed to publish the version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest with respect to this article.
Ethical approval
The study was approved by the Ethics Committee of the Faculty of Medicine, Warsaw, Poland (approval code KB/227/2016 approved on 08-11-2016), the Ethics Committee of Institute of Hematology and Blood Transfusion, Warsaw, Poland (approval code 27/2016 approved on 12-09-2016), and the Ethics Committee of the Military Medical Institute, Warsaw, Poland (approval code 65/WIM/2016 approved on 19-10-2016).
Consent to participate
All samples were analyzed anonymously. Blood samples were taken after informed consent for the procedures. Research was performed in compliance with the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deręgowska, A., Pępek, M., Solarska, I. et al. The interplay between telomeric complex members and BCR::ABL1 oncogenic tyrosine kinase in the maintenance of telomere length in chronic myeloid leukemia. J Cancer Res Clin Oncol 149, 7103–7112 (2023). https://doi.org/10.1007/s00432-023-04662-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04662-w