Abstract
Purpose
ERAS is a holistic and multidisciplinary pathway that incorporates various evidence-based interventions to accelerate recovery and improve clinical outcomes. However, evidence on cost benefit of ERAS in pancreaticoduodenectomy remains scarce. This review aimed to investigate cost benefit, compliance, and clinical benefits of ERAS in pancreaticoduodenectomy.
Methods
A comprehensive literature search was conducted on Medline, Embase, PubMed, CINAHL and the Cochrane library to identify studies conducted between 2000 and 2021, comparing effect of ERAS programmes and traditional care on hospital cost, length of stay (LOS), complications, delayed gastric emptying (DGE), readmission, reoperation, mortality, and compliance.
Results
The search yielded 3 RCTs and 28 cohort studies. Hospital costs were significantly reduced in the ERAS group (SMD = − 1.41; CL, − 2.05 to − 0.77; P < 0.00001). LOS was shortened by 3.15 days (MD = − 3.15; CI, − 3.94 to − 2.36; P < 0.00001) in the ERAS group. Fewer patients in the ERAS group had complications (RR = 0.83; CI, 0.76–0.91; P < 0.0001). Incidences of DGE significantly decreased in the ERAS group (RR = 0.72; CI, 0.55–0.94; P = 0.01). The number of deaths was fewer in the ERAS group (RR = 0.76; CI, 0.58–1.00; P = 0.05).
Conclusion
This review demonstrated that ERAS is safe and feasible in pancreaticoduodenectomy, improves clinical outcome such as LOS, complications, DGE and mortality rates, without changing readmissions and reoperations, while delivering significant cost savings. Higher compliance is associated with better clinical outcomes, especially LOS and complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1997, (Kehlet May 1997) introduced a multimodal approach to manage postoperative complications, which later evolved into enhanced recovery after surgery (ERAS). ERAS is a holistic and multidisciplinary pathway that incorporate various evidence-based interventions to accelerate recovery and reduce length of stay (LOS). Furthermore, it aimed to standardise care for patients undergoing specific procedures, with a view to improving clinical outcomes. ERAS was initially implemented in colorectal surgery. Due to its success, it was quickly adopted in other surgical specialities.
Pancreatic surgery is traditionally associated with high mortality and complication rates. Few decades ago, mortality in pancreatic surgery was as high as 25%, but has now fallen to under 5% owing to recent advances in diagnosis, surgical techniques and improvement in perioperative care management (Gooiker et al. 2014). However, complications tend to remain very high, ranging between 40 and 60% (Lermite, et al. 2013; Kunstman et al. 2019). Complications such as postoperative pancreatic fistula and delayed gastric emptying (DGE) are identified as the primary causes of delayed recovery which often require further radiological or surgical interventions (Zhang et al. 2020).
The past decade has seen various ERAS guidelines published for multiple surgical specialties including colorectal, cardiac, orthopaedic, breast and gastrointestinal surgery. The first ERAS guidelines for pancreatoduodenectomy were published by the ERAS society in 2012 (Lassen et al. 2012). The updated guidelines published in 2020 contain 27 elements, covering the three phases of perioperative care (preoperative, intra-operative and postoperative), including preoperative education, minimally invasive techniques, pain control and early mobilisation and feeding (Melloul et al. 2019).
The impact of ERAS has been widely studied in various surgical specialities including upper gastrointestinal surgeries with good results. In recent years, many studies have been published on the effect of ERAS in pancreatic surgery. These studies have demonstrated that implementation of the ERAS pathway in pancreatic surgery is safe and reduces LOS and complications without increasing mortality rates and readmissions. However, evidence on cost benefit of ERAS programmes in pancreatic surgery remains scarce. A recent meta-analysis of 27 studies demonstrated significant cost savings following the implementation of the ERAS pathway in liver surgery (Noba et al. 2020). To date, no meta-analysis has been conducted to evaluate the impact of ERAS in pancreaticoduodenectomy on hospital costs. The aim of this review is to investigate cost benefit, compliance and clinical benefits of ERAS in pancreaticoduodenectomy.
Methods
Search strategy
This review was conducted in compliance with PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines for systematic reviews and meta-analysis (Moher 2010). Multiple databases, (Medline, Embase, PubMed, CINAHL, and the Cochrane library), were searched to identify studies published between January 2000 and December 2021. The search was restricted to English language publications. A further search was conducted on the reference lists of relevant eligible studies and Systematic Reviews. The search terms such as ‘ERAS’, ‘FTS’, ‘Fast track’, ‘Enhanced recovery’, ‘Clinical pathway’, ‘Critical pathway’, ‘Accelerated recovery surgery’, ‘Pancreas’, ‘Pancreatic’, ‘Whipple’, ‘Pancreatectomy’, ‘Pancreatoduodenectomy’, ‘Pancreaticoduodenectomy’ were applied using Boolean operators (OR and AND).
Inclusion/exclusion criteria
Studies were eligible for inclusion if they met all of the following criteria (1) adult patients undergoing pancreaticoduodenectomy (2) compared ERAS to traditional care (3) reported at least one of the following outcomes: Hospital Costs, LOS, Complications, Compliance, Delayed Gastric Emptying (DGE), Mortality rates, Readmissions and Reoperations. Studies were excluded if they were non-elective or transplant patients, non-pancreaticoduodenectomy (PD), non-English and not comparing ERAS to traditional care.
Data extraction
Eligible studies and relevant data were retrieved and extracted by the first author. Data were extracted using a data extraction sheet agreed by all authors and were subsequently validated by other authors. Data extracted included; authors’ names, year of publication, study design, patient’ characteristics (ASA grade, age, sex and BMI), type of surgery, surgical techniques, outcomes measured, sample size, follow-up period and ERAS items.
Outcomes of interest
The primary outcomes for this systematic review were hospital costs. Secondary outcomes included: length of stay, compliance, complications, DGE, mortality, readmission and reoperation. LOS is defined by the total number of days a patient spent in the hospital prior to discharge.
Quality assessment
In line with the Cochrane Collaboration’s risk of bias tool, the quality of the Randomised Control Trials (RCTs) were assessed against the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective reporting (Higgins et al. 2011). See Fig. 1 for summary of risk of bias of RCTs. The methodological quality of the cohort studies were assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS) (Hartling et al. 2012). The NOS has a maximum of 9 stars (Selection 4 stars, Comparability 2 stars and Exposure 3 stars).
Statistical analysis
This review was conducted using Review Manager (RevMan) version 5.4 (Collaboration 2020). Risk ratio was used for all dichotomous variables, weight mean difference or weight standardised mean difference for continuous variables with 95% confidence interval (CI). Statistical significance level was set at p < 0.05. Statistical heterogeneity was assessed using a chi-squared test (χ2), I2 statistic. A P < 0.1 was considered to be a statistically significant heterogeneity. A fixed effect model was applied for pooling. Where there is substantial evidence of heterogeneity (I2 > 60%), a random effect model was applied instead. Using the method recommended by (Hozo et al. 2005), study data presented as medians and interquartile ranges were converted to mean and standard deviation (SD). Standard deviation from a study with similar sample size was used with the mean as suggested by (Furukawa et al. 2006). The presence of publication bias was assessed using Funnel plots.
Results
Search results
An initial search resulted in 835 studies. After inclusion/exclusion criteria were applied, 31 final studies were included in the meta-analysis. See Fig. 2 for the PRISMA flow chart.
Characteristics of included studies
A total of 5382 patients were included in this review (range between 41 and 635, per study), with 2776 patients in the ERAS group and 2606 patients in the traditional care group. Full details of the characteristics for included studies is shown in Table 1.
The number of ERAS items applied across the studies varied substantially. While, five studies did not provide lists of items utilised in their study (French et al. 2009), (Téoule et al. 2020). A detailed list of ERAS items utilised by individual studies is shown in Table 2. Three studies were RCTs (Deng et al. 2017), (Takagi et al. 2019), while the remaining studies were cohort studies (French et al. 2009), (Téoule et al. 2020; Joliat et al. 2015), (Hilal et al. 2013). The surgical approach was reported in six studies. Of these studies, four were open surgery (Hwang et al. 2019; Partelli et al. 2016; Braga et al. 2014; Hilal et al. 2013), one combined robotic and open surgery (Kowalsky et al. 2019), while the remaining study utilised a combination of open and laparoscopic approach (Nussbaum et al. 2015). Full details of characteristics for included studies is shown in Table 1.
Sensitivity analysis and publication bias
Funnel plots for LOS and readmission rates were used to assess publication bias as shown in Figs. 3, 4. The asymmetry of the funnel plots suggested no evidence of publication bias. In the presence of heterogeneity, a sensitivity analysis was conducted to test the reliability of the results.
Hospital costs
Ten studies evaluated hospital costs (3378 patients). Four of the studies measured hospital costs in US dollar (Takagi et al. 2019; Kennedy et al 2007; Kowalsky et al. 2019; Vanounou et al. 2007), two in Chinese yuan (Shao et al. 2015; Dai et al. 2017), two in euros (Joliat et al. 2015; Williamsson et al. 2015), one each in Canadian dollar (Kagedan et al. 2017) and South Korean won (Hwang et al. 2019). The pooled analysis suggested hospital costs were significantly lower in the ERAS group compared to the traditional care group (SMD = − 1.41; CL, − 2.05 to − 0.77; P < 0.00001). However, there was significant evidence of heterogeneity observed in the studies (χ2 = 389.50; df = 9; P < 0.00001; I2 = 98%). Similarly, in the subgroup analysis of studies conducted in different continents, hospital costs were lower in the ERAS group in studies conducted in North America (SMD = − 2.76; CL, − 4.54 to − 0.98; P = 0.002) and East Asia (SMD = − 0.35; CL, − 0.47 to − 0.23; P < 0.00001), while there was no difference in studies conducted in Europe (SMD = − 1.02; CL, − 2.18–0.14); P = 0.08). There was evidence of substantial heterogeneity in studies conducted in North America (χ2 = 257.00; df = 3; P < 0.00001; I2 = 99%) and Europe (χ2 = 18.84; df = 1; P < 0.0001; I2 = 95%). On the contrary, there no evidence of heterogeneity in studies conducted in Asia (χ2 = 1.93; df = 3; P = 0.59; I2 = 0%). There was a significant difference in hospital costs across the three continents (χ2 = 18.16; df = 3; P = 0.0004; I2 = 83.5%). See Fig. 5.
Length of stay
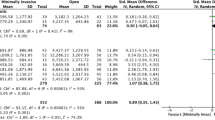
Length of stay was reported in all studies. Pooling of all results demonstrated a significant reduction in LOS in the ERAS group compared to the traditional care group (MD = − 3.15; CI, − 3.94 to − 2.36; P < 0.00001), with evidence of heterogeneity (χ2 = 513.70; df = 30; P < 0.00001; I2 = 94%). In addition, a subgroup analysis demonstrated a shorter LOS after implementation of ERAS in studies conducted in North America (MD = − 2.45; CI, − 3.42 to − 1.48; P < 0.00001), Europe (MD = − 2.23; CI, − 3.67 to − 0.79; P = 0.002) and Asia (MD = − 4.99; CI, − 7.57 to − 2.41; P = 0.0002). There was no significant difference in LOS in the three continents (χ2 = 4.56; df = 3; P = 0.21, I2 = 34.3%). See Fig. 6.
Complication rates
Twenty-five reported incidences of complications. Overall complications were reported in thirty-four studies (4454 patients). A total of 2417 patients experienced complications, 1101 patients in ERAS groups compared to 1316 in traditional care groups. One study reported no complication in both the ERAS and traditional groups (Nikfarjam et al. 2013). The meta-analysis revealed a significant reduction in rates of complication in the ERAS group (RR = 0.83; CI, 0.76–0.91; P < 0.0001), however, there was evidence of substantial heterogeneity (χ2 = 60.31; df = 23; P < 0.0001; I2 = 62%). Eighteen studies provided data on major complications (2608 patients). 553 patients had major complications, 268 patients in ERAS vs 285 patients in traditional care. Pooling the results demonstrated that major complications were comparable in both groups (RR = 0.96; CL, 0.83–1.11; P = 0.57), with no significant evidence of heterogeneity (χ2 = 24.03, df = 17; P = 0.12; I2 = 29%). See Figs. 7, 8.
Delayed gastric emptying (DGE)
Twenty-six studies supplied data on DGE (4734 patients). Of these, three studies recorded DGE according to their own centre definition (Kennedy et al. 2007; Su et al. 2017; Braga et al. 2014), two studies did not state how DGE was evaluated (Sutcliffe et al. 2015; Tremblay St-Germain et al. 2017), while the remaining studies defined DGE according to the International Study Group of Pancreatic Surgery (ISGPS) (Wente et al. 2007). Cases of DGE were recorded in 774 patients, with 322 being in the ERAS group compared to 452 in traditional care. The pooled analysis demonstrated significantly fewer cases of DGE in the ERAS group (RR = 0.72; CI, 0.55–0.94; P = 0.01). However, there was evidence of substantial heterogeneity (χ2 = 79.42; df = 25; P < 0.00001; I2 = 69%). See Fig. 9.
Mortality rates
Mortality rates were reported in 30 studies (5341 patients). Eight studies reported zero mortality (Deng et al. 2017), (Takagi et al. 2019; Su et al. 2017; Williamsson et al. 2015; Zhu et al. 2020; Dai et al. 2017; Hilal et al. 2013). In one study (Shao et al. 2015), mortality rates were substantially higher than normal (12% in the ERAS group vs 17.1% in the traditional care group), this was likely due to long-term follow up in the study (ranged from 1.3 to 48 months). A total of 192 deaths occurred in the studies, 84 patients in the ERAS, compared to 108 in the traditional care. On pooling the results, the number of deaths was significantly lower in the ERAS group (RR = 0.76; CI, 0.58–1.00; P = 0.05) and there was no evidence of heterogeneity (χ2 = 10.12; df = 21; P = 0.98; I2 = 0%). See Fig. 10.
Readmission rates
Twenty-eight studies supplied data for readmissions (5101 patients). Following hospital discharge, 561 patients were readmitted within 30 days (297 in ERAS compared to 264 in traditional care). There was no difference in ERAS and traditional care after pooling the results (RR = 1.07; CI, 0.91–1.25; P = 0.40), with no evidence of heterogeneity observed (χ2 = 18.46; df = 25; P = 0.82; I2 = 0%). See Fig. 11.
Reoperation rates
Reoperation rates were reported in fourteen studies (2419 patients). A total of 166 patients had to be reoperated, 81 patients in ERAS and 85 in traditional care. A pooled analysis found both groups to have similar reoperation rates (RR = 0.98; CI, 0.73–1.31; P = 0.88). There was no evidence of heterogeneity (χ2 = 9.55, df = 13; P = 0.73; I2 = 0%). See Fig. 12.
Compliance
Six studies evaluated overall compliance to key elements of the ERAS pathway. Two of these studies compared rates of compliance to ERAS items between ERAS group and traditional care group. Compliance was significantly higher in ERAS group, ranging 81.2–90.3% in ERAS group compared to 34.9–43.8% in traditional care. The remaining four studies did not compare compliance between the two groups. (Joliat et al. 2015) reported 70% rates of compliance in the ERAS group, while (Van der Kolk et al. 2017) reported 80% compliance during intensive care and 60% for the surgical ward period, respectively. Similarly, Zouros et al. (Zouros et al. 2016) found compliance to 13 key ERAS items to be > 74%, with 100% compliance in five of the 13 key elements. However, (Braga et al. 2014) recorded the lowest compliance (ranged between 38 and 66%). Two studies investigated correlation between compliance and clinical outcomes. In these studies, higher compliance was associated with fewer complications (Zouros et al. 2016; Braga et al. 2014) and shorter Length of stay (Zouros et al. 2016).
Discussion
Pancreatoduodenectomy is the most common treatment for pancreatic cancer. However, it remains one of the most complex and challenging procedures (Navarro 2017). Despite the significant improvement in outcomes such as mortality rate, complications remain as high as 60% (Lermite et al. 2013; Kunstman et al. 2019) and are the main reason for delayed discharge (Zhang et al. 2020).
This present meta-analysis included a total of 31 studies and 5382 patients making it the largest study to date on this topic. Previous systematic reviews and meta-analyses have concluded that implementation of ERAS pathways may reduce length of hospital stay and overall complications in pancreatoduodenectomy without increasing rates of mortality and readmission (Coolsen et al. 2013), (Wang et al. 2020).
With regard to the primary outcome, this review pooled sufficient data to investigate the impact of ERAS on hospital costs in pancreaticoduodenectomy. Three previous reviews included data on hospital costs in their analysis (Coolsen et al. 2013; Kagedan et al. 2015; Xiong et al. 2016), however, these data were not pooled. By contrast, this review included 10 studies on hospital costs, making it the first meta-analysis to confirm that implementation of ERAS can achieve significant cost savings in pancreatoduodenectomy. The reduction in hospital costs was also observed in the subgroup analysis of studies conducted in North America and East Asia, thereby strengthening the findings of this review. However, hospital costs varied significantly. This variation may be due to how medical costs are calculated from one centre to another. This emphasises the need for a standardised method of reporting medical costs.
Regarding secondary outcomes, this review found a significant reduction in length of stay of 3.15 days following implementation of ERAS protocols; a finding that is consistent with previous reviews on pancreatic surgery (Sun et al. 2020), (Wang et al. 2020). However, it is worth noting the presence of heterogeneity in the LOS. Despite conducting a sensitivity analysis, the heterogeneity still existed. The presence of heterogeneity could be due to several reasons, for example, how length of stay is calculated. Some studies reported LOS as either total LOS or postoperative LOS, whilst the majority of the studies did not state whether LOS was calculated as total length of stay or postoperative length of stay. Furthermore, the model of healthcare delivery differs significantly from one country to another, along with cultural ethos. For example, in countries such as the United Kingdom, it is a standard practice for a postoperative patient to be discharged from hospital to continue rehabilitation in the community. Whereas this practice is rare in many other countries and may not be affordable to patients without health insurance (Xiong, et al. 2016).This review also demonstrated that ERAS reduces cases of overall complications and delayed gastric emptying (DGE). A separate analysis was conducted to investigate the impact of ERAS on major complications. This finding was consistent with previous reviews (Bin Ji et al. 2018; Sun et al. 2020), major complications did not change in the ERAS group.
Contrary to previous reviews, mortality rates were significantly lower in the ERAS group. However, this result was swayed in favour of ERAS by a study that conducted a long-term follow-up (Shao et al. 2015). When this study was excluded from the meta-analysis, there was no significant difference between both groups (RR = 0.80; 0.55–1.17; P = 0.25). Therefore, the long-term impact of the ERAS pathway should be investigated further in high-quality randomised control trials (RCTs). Meanwhile, introduction of an ERAS pathway did not reduce readmissions and reoperations compared to traditional care.
The numbers of ERAS items utilised across all studies varied significantly. None of the studies included in this review applied all 27 items in the ERAS guidelines for pancreatoduodenectomy (Lassen et al. 2012; Melloul et al. 2019), with some studies using as little as six items. This is likely to be due to most studies being conducted before the first ERAS guidelines for pancreatoduodenectomy were published in 2012 (Lassen et al. 2012). The key ERAS items identified were preoperative education and counselling, minimum fasting and administration of carbohydrate drinks prior to surgery, epidural analgesia, intravenous fluids restriction, prevention of hypothermia, early removal of urinary catheters and abdominal drains, early oral intake, early mobilisation, early commencement of oral analgesia and prevention of postoperative nausea and vomiting (PONV). Early oral intake and early mobilisation were the most common interventions and were implemented in thirty-one studies, while preoperative carbohydrate drinks were the least implemented intervention and were on only administered 2–3 h prior to surgery in thirteen studies.
Most of the studies included did not investigate compliance to the ERAS pathway. When investigated, compliance rates were found to be significantly higher in the ERAS group in studies comparing compliance between the two groups (Takagi et al. 2019; Su et al. 2017). In studies that investigated compliance to key elements of ERAS in the ERAS group (Zouros et al. 2016; Braga et al. 2014), poor compliance was more prevalent in the postoperative ERAS elements particularly, oral analgesia, resumption of free fluids and normal diet and removal of abdominal drain and nasogastric tube. Moreover, patients with poor compliance experienced higher incidence of complications and prolonged hospital stay. Hence, flagging patients with poor compliance to key postoperative ERAS items may allow early identification of patients group that require additional care or further investigation.
It is worth mentioning the limitations in this current review.
-
(1)
The presence of heterogeneity was observed in hospital costs, LOS, overall complications and DGE. Where there was evidence of heterogeneity, sensitive analyses were conducted to investigate the influence of a single study by eliminating a study at each round. Despite this analysis, it was not possible to reduce the presence of heterogeneity below substantial level. Although, a random effect was used where heterogeneity could not be eliminated, however, it is not certain how this would have impacted the reliability of findings of this review.
-
(2)
None of the studies included in this review adopted current ERAS guidelines, which may have contributed to significant evidence of heterogeneity. A future study solely based on current ERAS guidelines on pancreaticoduodenectomy.
-
(3)
Most of the studies do not specify surgical approach applied in the surgeries; therefore, this review was unable to reach a conclusion on the additional benefits of minimally invasive approach in ERAS protocols. A future high quality RCTs is recommended to obtain this useful information.
Conclusion
This current review demonstrated that the implementation of ERAS is safe and feasible in pancreaticoduodenectomy, improves clinical outcomes such as length of stay, complications, DGE and mortality rates, without changing readmission and reoperation rates, while delivering significant cost savings. High levels of compliance can be achieved in ERAS and is associated with better clinical outcomes especially LOS and complications.
Evidently, successful implementation of ERAS is dependent on compliance to key elements. Therefore, early identification of patients with poor compliance may ensure this group are given additional care to maximise clinical outcomes.
References
Ahanatha Pillai S, Palaniappan R, Pichaimuthu A, Rajendran KK, Sathyanesan J, Govindhan M (2014) Feasibility of implementing fast-track surgery in pancreaticoduodenectomy with pancreaticogastrostomy for reconstruction - A prospective cohort study with historical control. Int J Surg 12(9):1005–1009. https://doi.org/10.1016/j.ijsu.2014.07.002
Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V (2008) Fast-track recovery programme after pancreaticoduodenectomy reduces delayed gastric emptying. Br J Surg 95(11):1387–1393. https://doi.org/10.1002/bjs.6324
Bin Ji H, Zhu WT, Wei Q, Wang XX, Bin Wang H, Chen QP (2018) Impact of enhanced recovery after surgery programs on pancreatic surgery: a meta-analysis. World J Gastroenterol 24(15):1666–1678. https://doi.org/10.3748/wjg.v24.i15.1666
Braga M et al (2014) Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg 38(11):2960–2966. https://doi.org/10.1007/s00268-014-2653-5
Coolsen MME, Van Dam RM, Van Der Wilt AA, Slim K, Lassen K, Dejong CHC (2013) Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 37(8):1909–1918. https://doi.org/10.1007/s00268-013-2044-3
Coolsen MME, Van Dam RM, Chigharoe A, Damink SWMO, Dejong CHC (2014) Improving outcome after pancreaticoduodenectomy: experiences with implementing an enhanced recovery after surgery (ERAS) program. Dig Surg 31(3):177–184. https://doi.org/10.1159/000363583
Dai J, Jiang Y, Fu D (2017) Reducing postoperative complications and improving clinical outcome: Enhanced recovery after surgery in pancreaticoduodenectomy – A retrospective cohort study. Int J Surg 39:176–181. https://doi.org/10.1016/j.ijsu.2017.01.089
Deng X et al (2017) Modified protocol for enhanced recovery after surgery is beneficial for Chinese cancer patients undergoing pancreaticoduodenectomy. Oncotarget 8(29):47841–47848. https://doi.org/10.18632/oncotarget.18092
French JJ, Mansfield SD, Jaques K, Jaques BC, Manas DM, Charnley RM (2009) Fast-track management of patients undergoing proximal pancreatic resection. Ann R Coll Surg Engl 91(3):201–204. https://doi.org/10.1308/003588409X391893
Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N (2006) Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 59(1):7–10. https://doi.org/10.1016/j.jclinepi.2005.06.006
Gooiker GA et al (2014) Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg 101(8):1000–1005. https://doi.org/10.1002/bjs.9468
Hartling L et al (2012) “Validity and inter-rater reliability testing of quality assessment instruments.” Agency Healthc Res Qual
Higgins JPT et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. https://doi.org/10.1136/bmj.d5928
Hilal MA et al (2013) Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology 13(1):58–62. https://doi.org/10.1016/j.pan.2012.11.312
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5(1):1–10. https://doi.org/10.1186/1471-2288-5-13
Hwang DW et al (2019) Effect of enhanced recovery after surgery program on pancreaticoduodenectomy: a randomized controlled trial. J Hepatobiliary Pancreat Sci 26(8):360–369. https://doi.org/10.1002/jhbp.641
Joliat GR et al (2015) Cost-benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg 102(13):1676–1683. https://doi.org/10.1002/bjs.9957
Kagedan DJ, Ahmed M, Devitt KS, Wei AC (2015) Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB 17(1):11–16. https://doi.org/10.1111/hpb.12265
Kagedan DJ, Devitt KS, Tremblay St-Germain A, Ramjaun A, Cleary SP, Wei AC (2017) The economics of recovery after pancreatic surgery: detailed cost minimization analysis of an enhanced recovery program. HPB 19(11):1026–1033. https://doi.org/10.1016/j.hpb.2017.07.013
Kehlet H (1997) Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 78(5):606–617. https://doi.org/10.1093/bja/78.5.606
Kennedy EP et al (2007) Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution-the first step in multidisciplinary team building. J Am Coll Surg 204(5):917–923. https://doi.org/10.1016/j.jamcollsurg.2007.01.057
Kobayashi S et al (2014) Perioperative care with fast-track management in patients undergoing pancreaticoduodenectomy. World J Surg 38(9):2430–2437. https://doi.org/10.1007/s00268-014-2548-5
Kowalsky SJ et al (2019) A combination of robotic approach and ERAS pathway optimizes outcomes and cost for pancreatoduodenectomy. Ann Surg 269(6):1138–1145. https://doi.org/10.1097/SLA.0000000000002707
Kuemmerli C et al (2022) Impact of enhanced recovery protocols after pancreatoduodenectomy: meta-analysis. Br J Surg. https://doi.org/10.1093/bjs/znab436
Kunstman JW et al (2019) Outcomes after pancreatectomy with routine pasireotide use. J Am Coll Surg 228(2):161-170.e2. https://doi.org/10.1016/j.jamcollsurg.2018.10.018
Lassen K et al (2012) Guidelines for perioperative care for pancreaticoduodenectomy: enhanced recovery after surgery (ERAS®) society recommendations. Clin Nutr 31(6):817–830. https://doi.org/10.1016/j.clnu.2012.08.011
Lermite E et al (2013) Complications after pancreatic resection: diagnosis, prevention and management. Clin Res Hepatol Gastroenterol 37(3):230–239. https://doi.org/10.1016/j.clinre.2013.01.003
Melloul E et al (2020) Guidelines for perioperative care for pancreatoduodenectomy: enhanced recovery after surgery (ERAS) recommendations 2019. World J Surg 44(7):2056–2084. https://doi.org/10.1007/s00268-020-05462-w
Moher D et al (2012) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. https://doi.org/10.1016/j.ijsu.2011.10.001
Morales Soriano R et al (2015) Outcomes of an enhanced recovery after surgery programme for pancreaticoduodenectomy. Cir Esp 93(8):509–515. https://doi.org/10.1016/j.ciresp.2015.04.009
Navarro S (2017) The art of pancreatic surgery. Past, present and future. The history of pancreatic surgery. Gastroenterol y Hepatol. https://doi.org/10.1016/j.gastre.2017.10.010
Nikfarjam M et al (2013) A fast track recovery program significantly reduces hospital length of stay following uncomplicated pancreaticoduodenectomy. J Pancreas 14(1):63–70. https://doi.org/10.6092/1590-8577/1223
Noba L, Rodgers S, Chandler C, Balfour A, Hariharan D, Yip VS (2020) Enhanced recovery after surgery (ERAS) reduces hospital costs and improve clinical outcomes in liver surgery: a systematic review and meta-analysis. J Gastrointest Surg 24(4):918–932. https://doi.org/10.1007/s11605-019-04499-0
Nussbaum DP et al (2015) A standardized care plan is associated with shorter hospital length of stay in patients undergoing pancreaticoduodenectomy. J Surg Res 193(1):237–245. https://doi.org/10.1016/j.jss.2014.06.036
Partelli S et al (2016) Evaluation of an enhanced recovery protocol after pancreaticoduodenectomy in elderly patients. HPB 18(2):153–158. https://doi.org/10.1016/j.hpb.2015.09.009
Shah OJ, Bangri SA, Singh M, Lattoo RA, Bhat MY, Khan FA (2016) Impact of centralization of pancreaticoduodenectomy coupled with fast track recovery protocol: a comparative study from India. Hepatobiliary Pancreat Dis Int 15(5):546–552. https://doi.org/10.1016/S1499-3872(16)60093-0
Shao Z, Jin G, Ji W, Shen L, Hu X (2015) The role of fast-track surgery in pancreaticoduodenectomy: a retrospective cohort study of 635 consecutive resections. Int J Surg 15:129–133. https://doi.org/10.1016/j.ijsu.2015.01.007
Su W et al (2017) A hospital-to-home evaluation of an enhanced recovery protocol for elective pancreaticoduodenectomy in China. Medicine (united States). https://doi.org/10.1097/MD.0000000000008206
Sun YM, Wang Y, Mao YX, Wang W (2020) The safety and feasibility of enhanced recovery after surgery in patients undergoing pancreaticoduodenectomy: an updated meta-analysis. Biomed Res Int. https://doi.org/10.1155/2020/7401276
Sutcliffe RP et al (2015) Implementation of an enhanced recovery pathway after pancreaticoduodenectomy in patients with low drain fluid amylase. World J Surg 39(8):2023–2030. https://doi.org/10.1007/s00268-015-3051-3
Takagi K et al (2019) Effect of an enhanced recovery after surgery protocol in patients undergoing pancreaticoduodenectomy: a randomized controlled trial. Clin Nutr 38(1):174–181. https://doi.org/10.1016/j.clnu.2018.01.002
Téoule P et al (2020) Influence of clinical pathways on treatment and outcome quality for patients undergoing pancreatoduodenectomy? A retrospective cohort study. Asian J Surg 43(8):799–809. https://doi.org/10.1016/j.asjsur.2019.10.003
Tremblay St-Germain A et al (2017) The impact of a clinical pathway on patient postoperative recovery following pancreaticoduodenectomy. HPB 19(9):799–807. https://doi.org/10.1016/j.hpb.2017.04.015
The Cochrane Collaboration (2020) “Review Manager (Revman) 5.4.” The Cochrane Collaboration, Copenhagen 1–43
van der Kolk M et al (2017) Implementation and evaluation of a clinical pathway for pancreaticoduodenectomy procedures: a prospective cohort study. J Gastrointest Surg 21(9):1428–1441. https://doi.org/10.1007/s11605-017-3459-1
Vanounou T, Pratt W, Fischer JE, Vollmer CM, Callery MP (2007) Deviation-based cost modeling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg 204(4):570–579. https://doi.org/10.1016/j.jamcollsurg.2007.01.025
Wang XY, Cai JP, Huang CS, Huang XT, Yin XY (2020) Impact of enhanced recovery after surgery protocol on pancreaticoduodenectomy: a meta-analysis of non-randomized and randomized controlled trials. HPB. https://doi.org/10.1016/j.hpb.2020.07.001
Wente MN et al (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 142(5):761–768. https://doi.org/10.1016/J.SURG.2007.05.005
Williamsson C, Karlsson N, Sturesson C, Lindell G, Andersson R, Tingstedt B (2015) Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br J Surg 102(9):1133–1141. https://doi.org/10.1002/bjs.9856
Williamsson C, Karlsson T, Westrin M, Ansari D, Andersson R, Tingstedt B (2019) Sustainability of an enhanced recovery program for pancreaticoduodenectomy with pancreaticogastrostomy. Scand J Surg 108(1):17–22. https://doi.org/10.1177/1457496918772375
Xiong J et al (2016) Enhanced recovery after surgery program in patients undergoing pancreaticoduodenectomy a PRISMA-compliant systematic review and meta-analysis. Medicine (united States) 95(18):3497. https://doi.org/10.1097/MD.0000000000003497
Zhang XY et al (2020) Factors associated with failure of enhanced recovery after surgery program in patients undergoing pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int 19(1):51–57. https://doi.org/10.1016/j.hbpd.2019.09.006
Zhu J et al (2020) Enhanced recovery after surgery pathways benefit patients with soft pancreatic texture following pancreaticoduodenectomy. Am J Surg 219(6):1019–1023. https://doi.org/10.1016/j.amjsurg.2019.08.002
Zouros E, Liakakos T, MacHairas A, Patapis P, Agalianos C, Dervenis C (2016) Improvement of gastric emptying by enhanced recovery after pancreaticoduodenectomy. Hepatobiliary Pancreat Dis Int 15(2):198–208. https://doi.org/10.1016/S1499-3872(16)60061-9
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization of the study: LN, SR, LD, CC, DH and VY. Literature search, Data extraction and Data analysis: LN and Drafting of manuscript and critical revision of the work: LN, SR, LD, CC, DH and VY. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent and ethical approval
Not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noba, L., Rodgers, S., Doi, L. et al. Costs and clinical benefits of enhanced recovery after surgery (ERAS) in pancreaticoduodenectomy: an updated systematic review and meta-analysis. J Cancer Res Clin Oncol 149, 6639–6660 (2023). https://doi.org/10.1007/s00432-022-04508-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04508-x