Abstract
Purpose
In recent years, incidence of vulvar cancer has been on the rise, whereas therapeutic options are still restricted. Therefore, new prognosticators and therapeutic targets are essential. Chronic inflammation plays an important role in carcinogenesis and COX-2, and its product prostaglandin E2 and its receptors EP1–4 are known to be important mediators in cancer initiation and progression.
Methods
EP1 expression in vulvar cancer specimens (n = 129) was investigated via immunohistochemistry and evaluated using the well-established immunoreactive score (IRS). Subsequently, the values were correlated with clinicopathological parameters.
Results
Our analysis did not reveal EP1 expression as a negative prognostic factor in overall and disease-free survival. However, in the subgroup of patients with lymph-node metastasis, overall survival was significantly shorter in tumors with high EP1 expression. Moreover, EP1 expression correlated positively with good differentiation of the tumor, but not with p16 status or COX-2 expression.
Conclusions
This study shed first light on EP1 expression in vulvar carcinoma. EP1 expression correlated significantly with the grading of the tumor, suggesting that it influences cell differentiation. Further research on EP1 signaling may lead to a deeper understanding of the molecular mechanisms of carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vulvar carcinoma is a relatively rare disease, being the fifth most common gynecological tumor worldwide (International Agency for Research on Cancer 2020). Squamous cell carcinoma (SCC) is its most common histologic subgroup, representing around 90% of all tumors of the vulva (Kang et al. 2017). Incidence rates have been on the rise in recent years, possibly caused partly due to an increasing prevalence of human papilloma virus (HPV) infection likely promoted by a social change in sexual behavior (Bray et al. 2020). The HPV-related pathway leading to the development of around 40% of all vulvar squamous cell carcinoma (VSCC) can be distinguished from an HPV independent pathway often associated with chronic inflammatory dermatological diseases such as lichen sclerosis or lichen planus (Carlson et al. 1998; Regauer et al. 2014; Smith et al. 2009). Radical local surgical resection often accompanied by lymphonodectomy and adjuvant radiotherapy is the predominantly applied therapy for both tumor entities, while systemic therapies are only marginally administered resulting from a lack of randomized controlled trials (Mahner et al. 2015). Despite limited literature regarding long-term effects of vulvar cancer therapy, radical surgery often involving large resection areas up to vulvectomy can lead to reduced quality of life with impaired sexual function, lymph edema, or urinary difficulties (Gitas et al. 2021; Pilger et al. 2012; Ramaseshan et al. 2018). Although HPV vaccination gives the opportunity for primary prevention of a smaller proportion of VSCC, further risk factors such as smoking habits and an aging population additionally support the need for new predictive biomarkers and target-based therapies to ameliorate the clinical outcome for patients, especially in late stages of the disease (Daling et al. 1992; Joura et al. 2007). Currently, possible therapy targets are vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) (Mahner et al. 2015). VEGF is an important mediator for angiogenesis and when overexpressed has a negative impact on the prognosis of vulvar carcinoma patients (Obermair et al. 1996). EGFR is a transmembrane protein and belongs to the family of receptor tyrosine kinases. It is often overexpressed in a vulvar carcinoma, and is associated with decreased survival, high tumor stage, lymph-node metastasis, and HPV-negativity (Growdon et al. 2008; Woelber et al. 2012). There are few case reports describing clinical benefits from the treatment with monoclonal antibody Cetuximab (Bergstrom et al. 2015; Matsuzawa et al. 2016). Also, EGFR inhibitor Erlotinib showed overall clinical benefit in 67.5% of patients in a phase II clinical trial with 41 patients. Nevertheless, therapy with the EGFR inhibitor implicated significant grade 3/4 toxicity including allergic reaction, diarrhea with electrolyte abnormalities, ischemic colitis, and renal failure, and often is often accompanied by early resistance to therapy (Horowitz et al. 2012). Therefore, the search for new prognostic markers and possible targets is necessary.
As chronic inflammation is known to play an important role in cancer initiation, progression, angiogenesis, and metastasis, prostanoid metabolism presents an interesting research topic in different tumor entities (Shacter and Weitzman 2002). Previous studies investigated the function of cyclooxygenase enzyme 2 (COX-2), prostaglandin E2 (PGE2), and its receptors (EP) in many different tumor entities like colon cancer, prostate and lung cancers, as well as in breast cancer and gynecological tumors, such as ovarian, cervical, and endometrial cancers (Howe 2007; Wang and Dubois 2010a, 2010b; Ye et al. 2020). PGE2 is produced from arachidonic acid by cyclooxygenase enzyme 2 (COX-2) and mediates its effects through its specific G-protein coupled receptors EP1–EP4, whereas EP1 is studied the least (Ye et al. 2020). Affinity for PGE2 binding is substantially different between the EP receptor subtypes with the following rank order of affinities: EP3 > EP4 > > EP2 > EP1 (Abramovitz et al. 2000). Each receptor induces different signaling cascades, e.g., through the intracellular increase of Ca2+ and subsequently activation of protein kinase C (EP1) or the increase of secondary messenger cAMP levels and activation of protein kinase A (EP2 and EP4) (O’Callaghan and Houston 2015). EP1 is involved in several different signaling pathways. Previous research indicates the involvement of EP1 in an EGFR independent activation of the MAPK/ERK pathway in non-small cell cancer of the lung (Krysan et. al. 2005). Pan et al. demonstrated the regulatory role of EP1 on β1-integrin through the COX-2/EP1/MAPK/E2F-1 pathway (Pan et al. 2016). Moreover, EP1 seems to mediate enhanced matrix metalloproteinase-2 (MMP-2) expression by cAMP independent CREB phosphorylation, illustrating its importance in cancer invasion (Sun et. al. 2013). A study on hepatocellular carcinoma cells shows that EP1 promotes cell adhesion and migration by inducing phosphorylation of focal adhesion kinase (FAK) (Bai et al. 2013). Furthermore, in a in vivo study using a prostate cancer mouse model, mice that were treated with an EP1 antagonist showed a significantly lower incidence of cancer at simultaneously a higher percentage of apoptotic cells compared to control (Masato et. al. 2021).
The expression and function of COX-2, PGE 2, and EPs in vulvar cancer have been subject to little research to date. Results from previous studies identified combined cytoplasmatic COX-2 expression and EP4 expression as negative prognostic factors for survival in vulvar cancer patients. Furthermore, positive EP4 expression correlated significantly with higher FIGO stage and tumor size (Ansorge et al. 2021; Buchholz et al. 2021). The aim of this study is to examine the expression of EP1 in vulvar cancer via immunohistochemistry and to analyze its correlation with clinicopathologic variables and its effect on patients’ survival to possibly find new prognostic markers and possible therapeutic targets.
Methods
Patients collective
The patients’ collective was composed of 177 patients who were treated at the Department of Gynecology and Obstetrics of the Ludwig-Maximilians-University in Munich, Germany between 1990 and 2008. During surgery, collected tissue material was histopathological processed and specified. Munich Cancer Registry (MCR) from the Munich Tumor Centre (TZM—Munich Tumor Centre, Munich, Germany) provided the follow-up and survival data. For immunohistochemical staining, 157 of these 177 tissue samples were accessible. During microscopic analysis, another 28 samples were excluded, as the specific tissue slices did not contain cancer tissue. In the end, 129 patients were included in the statistical analysis. Patients’ median age was 69.5 years (range 20–96 years) and overall median survival was 7.03 years. Other relevant patient characteristics are displayed in Table 1.
Immunohistochemistry
Formalin fixated and paraffin-embedded samples were cut to 4 µm slices, before being mounted on SuperFrost Plus microscope slides (Menzel Glaeser, Braunschweig, Germany). Staining was conducted as previously similarly described (Buchholz et al. 2021). First, sample slides were deparaffined in xylol for 20 min and washed in 100% alcohol. To stop the activity of endogen peroxidases, samples were incubated in methanol with 3% H2O2 for 20 min. Afterward, the slides were rehydrated in descending alcohol (100%, 70%, and 50%) and washed with distilled water. In the use of a pressure cooker, samples were heated in sodium citrate buffer (pH = 6.0) (0.1 M citric acid and 0.1 M sodium citrate in distilled water) to unmask antigens, which agglomerate during formalin fixation. After cooling and washing in PBS, slides were prepared with a blocking solution (Power Block, Biogenex Laboratories, Fremont, CA, USA) for 5 min to saturate electrostatic charges and avoid nonspecific hydrophobic binding of the primary antibodies. In the next step, the primary anti-EP1 antibody dilution (1:300 in PBS) (polyclonal rabbit IgG; AB217925, Abcam, Cambridge, UK) was applied and the slides were incubated for 16 h at 4 °C in a humidity chamber. ZytoChem Plus HRP Polymer System (Zytomed, Berlin, Germany) was used for detection via secondary complex. It contained the application of a post-block reagent for 20 min and an HRP-polymer for 30 min thereafter, both at room temperature in the humidity chamber. In the end, coloration of the substrate was catalyzed by the chromogen diaminobenzidine (Dako, Hamburg, Germany) and stopped with H2O. In a last step, slides were counterstained with hemalaun for 2 min and washed in ascending alcohol and covered with glass. EP1 expression was evaluated with a Leitz microscope (Wetzlar, Germany) using the well-established semiquantitative immunoreactivity score (IRS). Therefore, a product of two factors is formed: the intensity of the staining (0 = no, 1 = weak, 2 = moderate, and 3 = strong staining) multiplied by the percentage of stained cells (0 = no staining, 1 = 10% positive cells, 2 = 11–50% positive cells, and 3 = 50% positive cells) (Remmele and Stegner 1987). Ultimately, the IRS score of the samples was correlated with clinicopathological parameters and groups with high and low EP1 expression were compared for progression-free and overall survival. Placenta tissue from the LMU Department of Gynecology and Obstetrics in Munich was utilized as negative and positive control.
Statistical analysis
Data analysis was performed with the Statistical Product and Service Solutions 28 (PASW Statistic, SPSS Inc., IBM, Chicago, IL, USA). Spearmen’s test was used to test for correlations between immunohistochemically staining and clinicopathological parameters. Nonparametric tests (Mann–Whitney U) were used for group comparisons regarding the IRS of the prostaglandin receptors between independent clinical and pathological subgroups and are displayed as boxplot graphs. Survival times were analyzed by Kaplan–Meier curves and log-rank testing (Mantel–Cox). Cut-off points were acquired by the receiver operator curve (ROC). We considered p values ≤ 0.05 as statistically significant.
Results
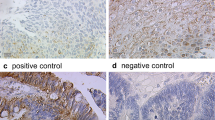
We achieved effectual EP1 staining from 129 patients. Cytosolic EP1 (IRS ≥ 1) staining was found in 86.0% (111/129 cases) of cases. High expression (IRS 9–12) was found in 6.2% of specimens, compared to moderate expression (IRS 6–8) in 22.5%, weak expression (IRS 3–4) in 29.5%, and no expression (IRS 0–2) in 41.9% (Fig. 1). In contrast to vulvar cancer specimens, no EP1 expression was found in the adjacent benign tissue of the vulva.
Illustration of different EP1 expression: a, b Vulvar cancer with high EP1 expression (IRS = 12). c, d Vulvar cancer with low EP1 expression (IRS = 1–2). e, f Adjacent benign vulva tissue shows no EP1 expression. Magnification and scale bars (a, c, e) × 25 with scale bar representing 100 µm and (b, d, f) × 40 with scale bar representing 50 µm
Correlation of enhanced EP1 expression with shorter overall survival in lymph-node positive patients
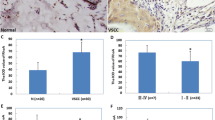
Regarding all samples, we could not detect any association between EP1 expression and overall survival (p = 0.441) or progression-free survival (p = 0.680). However, in the subgroup of patients with lymph-node metastasis, patients with enhanced EP1 expression (IRS > 3) showed significantly worse overall survival than patients with low EP1 expression (IRS ≤ 3) (median estimate: 2.6 years vs. 8.6 years; p = 0.028) (Fig. 2).
Kaplan–Meier curves display overall and disease-free survival in patients with high and low cytoplasmatic EP1 expression. a, b Kaplan–Meier survival univariate analysis for the status IRS > 3 showed no significantly diverse overall (p = 0.680) or disease-free survival (p = 0.441) c In the subgroup of patients with lymph-node metastasis, a high EP1 expression (IRS > 3) survival univariate analysis showed significantly shorter overall survival (p = 0.028). d In the subgroup of patients without lymph-node metastasis, survival univariate analysis showed no significantly diverse overall survival regarding EP1 expression (p = 0.603)
In this study, multivariate analysis identified age at diagnosis (p = 0.006) and grading (p = 0.022) as independent prognostic factors for overall survival. Enhanced EP1 expression, nodal status, tumor size, FIGO classification, or p16 status did not prove to be independent prognosticators (Table 2).
Correlation of EP1-positive staining with clinicopathologic parameters
Spearmen’s test was used to inspect the correlation between EP1 expression and clinicopathological parameters. We could not detect correlations between EP1 and lymph-node status (p = 0.633), tumor size (p = 0.807), FIGO classification (p = 0.582), the histologic subtype (p = 0.523), p16 status (p = 0.463), or cytosolic COX-2 expression (p = 0.230).
In contrast, EP1 positivity correlated significantly with lower grading of the tumor (p = 0.031) (Fig. 3). Mann–Whitney U Test confirmed those significant differences in EP1 expression between low-grade (G1) (high expression) and high-grade (G3) (low expression) tumors (p = 0.047).
Discussion
This study shed first light on the expression of G-coupled prostaglandin receptor EP1 in vulvar carcinoma and analyzed its correlation with clinicopathologic parameters and survival. To date, EP1 expression has been described in numerous tumor entities, including breast cancer, endometrial cancer, colon cancer, and skin cancer (Gustafsson et al. 2007; Lee et al. 2005; Thorat et al. 2008; Zhu et al. 2018). Moreover, prostaglandin receptor EP1 is known to be involved in tumor initiation and progression, cell migration and invasion, and the adaption of cancer cells to hypoxia (O’Callaghan and Houston 2015). In comparison to the other EP receptors, the knowledge on EP1 in cancer metabolism is still limited and discussed controversially.
Our results revealed no influence of EP1 on overall or disease-free survival in vulvar cancer patients. These findings are analogous to a study by Zhu et al. They analyzed EP1 in a large collective of endometrial cancer (n = 140) and could also not detect any correlation with survival or clinicopathological parameters in endometrial cancer (Zhu et al. 2018). Nonetheless, in our subgroup of patients with lymph-node metastasis, patients with elevated EP1 expression in the tumor had a significantly shorter overall survival.
Since EP1 is known to be involved in cell migration and invasion, it could be possible that EP1 becomes upregulated in tumors during the invasive process of metastasis. Unfortunately, we could not be present during the tissue collecting process; consequently, it was not possible to save lymph-node tissue for the evaluation of EP1 in metastatic tissue.
In addition, we found a correlation of EP1 with tumor grading: well-differentiated tumors showed a significantly higher EP1 expression than poorly differentiated tumors. This result is supported by previous research of EP1 expression in SCC of the skin. Lee et al. found higher EP1 expression, especially in the supra-basal layers of the epidermis in SCC of the skin compared to healthy epidermal tissue (Lee et. al. 2005). EP1 seems to be an important mediator for the regulation of keratinocyte differentiation. This idea is supported by a study of Konger et al. in which they described an inhibition of keratinocyte differentiation after EP1-receptor antagonism in vitro and an enhanced expression of EP1 in well-differentiated SCC compared to poorly differentiated SCC. This regulatory effect seemed to be intact in squamous cell carcinoma of the skin, which is in line with the results from our study (Konger et al. 2009). Furthermore, statistical analysis did surprisingly not reveal any correlation between COX-2 expression [archive data from a previous study by Ansorge et al. (2021)] and EP1 expression. The affinity of EP1 for PGE2 is relatively low; therefore, one could expect an upregulation of COX-2 in EP1-positive tumor samples (Abramovitz et al. 2000). However, previous research showed a posttranslational negative feedback mechanism of EP1 on COX-2 by proteasomal degradation and showed on the other side increased levels of EP1 at COX-2 overexpression, both through transient complex formation (Haddad et al. 2012; Sood et al. 2014).
Finally, some limitations of this study are worth to be noted: on account of the retrospective approach of our study, only one semiquantitative method (immunohistochemistry) was used to evaluate EP1 expression in the patients collective. As we could not be present during the tissue collecting process, it was not possible to save lymph-node tissue for evaluation of EP1 expression and fresh tissue to apply further methods for the analysis of protein activity. Nevertheless, a strength of this study is the large size of the patient collective, considering the rarity of the disease. This study gave first insight in the expression of EP1 in vulvar cancer and its association with clinical data.
Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical issues.
Abbreviations
- cAMP:
-
Cyclic adenosine monophosphate
- COX-2:
-
Cyclooxygenase-2
- CREB:
-
CAMP response element-binding protein
- EGFR:
-
Epidermal growth factor receptor
- EP1:
-
Prostaglandin E2 receptor 1
- ERK:
-
Extracellular-signal regulated kinase
- FAK:
-
Focal adhesion kinase
- FIGO:
-
Fédération Internationale de Gynécologie et d’Obstétrique
- HPV:
-
Human papilloma virus
- IRS:
-
Immunoreactive score
- MAPK:
-
Mitogen-activated protein kinases
- MMP-2:
-
Matrix metalloproteinase-2
- PGE2:
-
Prostaglandin E2
- ROC:
-
Receiver-operating characteristic
- SCC:
-
Squamous cell carcinoma
- VEGF:
-
Vascular endothelial growth factor
- VSCC:
-
Vulvar squamous cell carcinoma
References
Abramovitz M, Adam M, Boie Y, Carrière M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM (2000) The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta 1483(2):285–293. https://doi.org/10.1016/s1388-1981(99)00164-x
Ansorge N, Dannecker C, Jeschke U, Schmoeckel E, Mayr D, Heidegger HH, Vattai A, Burgmann M, Czogalla B, Mahner S, Fuerst S (2021) Combined COX-2/PPARγ expression as independent negative prognosticator for vulvar cancer patients. Diagnostics (basel) 11(3):491. https://doi.org/10.3390/diagnostics11030491
Bai X, Wang J, Zhang L, Ma J, Zhang H, Xia S, Zhang M, Ma X, Guo Y, Rong R, Cheng S, Shu W, Wang Y, Leng J (2013) Prostaglandin E2 receptor EP1-mediated phosphorylation of focal adhesion kinase enhances cell adhesion and migration in hepatocellular carcinoma cells. Int J Oncol 42(5):1833–1841. https://doi.org/10.3892/ijo.2013.1859
Bergstrom J, Bidus M, Miles E, Allard J (2015) Use of cetuximab in combination with cisplatin and adjuvant pelvic radiation for stage IIIB vulvar carcinoma. Case Rep Obstet Gynecol 2015:139817. https://doi.org/10.1155/2015/139817
Bray F, Laversanne M, Weiderpass E, Arbyn M (2020) Geographic and temporal variations in the incidence of vulvar and vaginal cancers. Int J Cancer 147(10):2764–2771. https://doi.org/10.1002/ijc.33055
Buchholz A, Vattai A, Fürst S, Vilsmaier T, Kuhn C, Schmoeckel E, Mayr D, Dannecker C, Mahner S, Jeschke U, Heidegger HH (2021) EP4 as a negative prognostic factor in patients with vulvar cancer. Cancers (basel) 13(6):1410. https://doi.org/10.3390/cancers13061410
Carlson JA, Ambros R, Malfetano J, Ross J, Grabowski R, Lamb P, Figge H, Mihm MC Jr (1998) Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol 29(9):932–948. https://doi.org/10.1016/s0046-8177(98)90198-8
Daling JR, Sherman KJ, Hislop TG, Maden C, Mandelson MT, Beckmann AM, Weiss NS (1992) Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol 135(2):180–189. https://doi.org/10.1093/oxfordjournals.aje.a116270
Gitas G, Proppe L, Baum S, Kruggel M, Rody A, Tsolakidis D, Zouzoulas D, Laganà AS, Guenther V, Freytag D, Alkatout I (2021) A risk factor analysis of complications after surgery for vulvar cancer. Arch Gynecol Obstet 304(2):511–519. https://doi.org/10.1007/s00404-020-05949-w
Growdon WB, Boisvert SL, Akhavanfard S, Oliva E, Dias-Santagata DC, Kojiro S, Horowitz NS, Iafrate AJ, Borger DR, Rueda BR (2008) Decreased survival in EGFR gene amplified vulvar carcinoma. Gynecol Oncol 111(2):289–297. https://doi.org/10.1016/j.ygyno.2008.07.038
Gustafsson A, Hansson E, Kressner U, Nordgren S, Andersson M, Wang W, Lönnroth C, Lundholm K (2007) EP1-4 subtype, COX and PPAR gamma receptor expression in colorectal cancer in prediction of disease-specific mortality. Int J Cancer 121(2):232–240. https://doi.org/10.1002/ijc.22582
Haddad A, Flint-Ashtamker G, Minzel W, Sood R, Rimon G, Barki-Harrington L (2012) Prostaglandin EP1 receptor down-regulates expression of cyclooxygenase-2 by facilitating its proteasomal degradation. J Biol Chem 287(21):17214–17223. https://doi.org/10.1074/jbc.M111.304220
Horowitz NS, Olawaiye AB, Borger DR, Growdon WB, Krasner CN, Matulonis UA, Liu JF, Lee J, Brard L, Dizon DS (2012) Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecol Oncol 127(1):141–146. https://doi.org/10.1016/j.ygyno.2012.06.028
Howe LR (2007) Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res 9(4):210. https://doi.org/10.1186/bcr1678
International Agency for Research on Cancer (2020) Globocon 2020, cancer/vulva (C51). World Health Organization. https://www.gco.iarc.fr/today/data/factsheets/cancers/21-Vulva-fact-sheet.pdf. Accessed 9 Sep 2022
Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, Steben M, Jones RW, Bryan J, Taddeo FJ, Bautista OM, Esser MT, Sings HL, Nelson M, Boslego JW, Sattler C, Barr E, Paavonen J (2007) Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet 369(9574):1693–1702. https://doi.org/10.1016/s0140-6736(07)60777-6
Kang YJ, Smith M, Barlow E, Coffey K, Hacker N, Canfell K (2017) Vulvar cancer in high-income countries: increasing burden of disease. Int J Cancer 141(11):2174–2186. https://doi.org/10.1002/ijc.30900
Konger RL, Billings SD, Prall NC, Katona TM, Dasilva SC, Kennedy CR, Badve S, Perkins SM, Lacelle PT (2009) The EP1 subtype of prostaglandin E2 receptor: role in keratinocyte differentiation and expression in non-melanoma skin cancer. Prostaglandins Leukot Essent Fatty Acids 81(4):279–290. https://doi.org/10.1016/j.plefa.2009.05.025
Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohadwala M, Dubinett SM (2005) Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Can Res 65(14):6275–6281. https://doi.org/10.1158/0008-5472.CAN-05-0216
Lee JL, Kim A, Kopelovich L, Bickers DR, Athar M (2005) Differential expression of E prostanoid receptors in murine and human non-melanoma skin cancer. J Invest Dermatol 125(4):818–825. https://doi.org/10.1111/j.0022-202X.2005.23829.x
Mahner S, Prieske K, Grimm D, Trillsch F, Prieske S, von Amsberg G, Petersen C, Mueller V, Jaenicke F, Woelber L (2015) Systemic treatment of vulvar cancer. Expert Rev Anticancer Ther 15(6):629–637. https://doi.org/10.1586/14737140.2015.1037837
Masato M, Miyata Y, Kurata H, Ito H, Mitsunari K, Asai A, Nakamura Y, Araki K, Mukae Y, Matsuda T, Harada J, Matsuo T, Ohba K, Sakai H (2021) Oral administration of E-type prostanoid (EP) 1 receptor antagonist suppresses carcinogenesis and development of prostate cancer via upregulation of apoptosis in an animal model. Sci Rep 11(1):20279. https://doi.org/10.1038/s41598-021-99694-y
Matsuzawa M, Inozume T, Sano S, Ando N, Onuma T, Harada K, Kawamura T, Shimada S (2016) A case of recurrent squamous cell carcinoma of the vulva successfully treated by combination therapy with cetuximab and paclitaxel. Br J Dermatol 174(3):677–678. https://doi.org/10.1111/bjd.14188
Obermair A, Kohlberger P, Bancher-Todesca D, Tempfer C, Sliutz G, Leodolter S, Reinthaller A, Kainz C, Breitenecker G, Gitsch G (1996) Influence of microvessel density and vascular permeability factor/vascular endothelial growth factor expression on prognosis in vulvar cancer. Gynecol Oncol 63(2):204–209. https://doi.org/10.1006/gyno.1996.0307
O’Callaghan G, Houston A (2015) Prostaglandin E2 and the EP receptors in malignancy: possible therapeutic targets? Br J Pharmacol 172(22):5239–5250. https://doi.org/10.1111/bph.13331
Pan J, Yang Q, Shao J, Zhang L, Ma J, Wang Y, Jiang BH, Leng J, Bai X (2016) Cyclooxygenase-2 induced β1-integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F-1/FoxC2 signal pathway. Sci Rep 6:33823. https://doi.org/10.1038/srep33823
Pilger A, Richter R, Fotopoulou C, Beteta C, Klapp C, Sehouli J (2012) Quality of life and sexuality of patients after treatment for gynaecological malignancies: results of a prospective study in 55 patients. Anticancer Res 32(11):5045–5049
Ramaseshan AS, Felton J, Roque D, Rao G, Shipper AG, Sanses TVD (2018) Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J 29(4):459–476. https://doi.org/10.1007/s00192-017-3467-4
Regauer S, Reich O, Eberz B (2014) Vulvar cancers in women with vulvar lichen planus: a clinicopathological study. J Am Acad Dermatol 71(4):698–707. https://doi.org/10.1016/j.jaad.2014.05.057
Remmele W, Stegner HE (1987) Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 8(3):138–140
Shacter E, Weitzman SA (2002) Chronic inflammation and cancer. Oncology (Williston Park) 16(2):217–232
Smith JS, Backes DM, Hoots BE, Kurman RJ, Pimenta JM (2009) Human papillomavirus type-distribution in vulvar and vaginal cancers and their associated precursors. Obstet Gynecol 113(4):917–924. https://doi.org/10.1097/AOG.0b013e31819bd6e0
Sood R, Flint-Ashtamker G, Borenstein D, Barki-Harrington L (2014) Upregulation of prostaglandin receptor EP1 expression involves its association with cyclooxygenase-2. PLoS ONE 9(3):e91018. https://doi.org/10.1371/journal.pone.0091018
Sun B, Rong R, Jiang H, Zhang H, Wang Y, Bai X, Zhang M, Ma J, Xia S, Shu W, Zhang L, Leng J (2013) Prostaglandin E2 receptor EP1 phosphorylate CREB and mediates MMP2 expression in human cholangiocarcinoma cells. Mol Cell Biochem 378(1–2):195–203. https://doi.org/10.1007/s11010-013-1610-1
Thorat MA, Morimiya A, Mehrotra S, Konger R, Badve SS (2008) Prostanoid receptor EP1 expression in breast cancer. Mod Pathol 21(1):15–21. https://doi.org/10.1038/modpathol.3800970
Wang D, Dubois RN (2010a) Eicosanoids and cancer. Nat Rev Cancer 10(3):181–193. https://doi.org/10.1038/nrc2809
Wang D, DuBois RN (2010b) The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29(6):781–788. https://doi.org/10.1038/onc.2009.421
Woelber L, Hess S, Bohlken H, Tennstedt P, Eulenburg C, Simon R, Gieseking F, Jaenicke F, Mahner S, Choschzick M (2012) EGFR gene copy number increase in vulvar carcinomas is linked with poor clinical outcome. J Clin Pathol 65(2):133–139. https://doi.org/10.1136/jcp-2010-079806
Ye Y, Wang X, Jeschke U, von Schonfeldt V (2020) COX-2-PGE2-EPs in gynecological cancers. Arch Gynecol Obstet 301(6):1365–1375. https://doi.org/10.1007/s00404-020-05559-6
Zhu J, Mayr D, Kuhn C, Mahner S, Jeschke U, von Schönfeldt V (2018) Prostaglandin E2 receptor EP1 in healthy and diseased human endometrium. Histochem Cell Biol 149(2):153–160. https://doi.org/10.1007/s00418-017-1616-y
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors acknowledge financial support by the Friedrich-Baur-Stiftung (registration number 33/19) for Helene H. Heidegger 36/18.
Author information
Authors and Affiliations
Contributions
All authors analyzed and interpreted the data and read and approved the manuscript. AB participated in design and coordination of the study, and performed immunohistochemistry assays and analysis and the statistical analysis. CK performed technical assistance in immunohistochemistry assays and analysis. AB and HHH wrote the manuscript. AV, SF, TV, AZZ, CD, and SM carefully read the manuscript for important intellectual content. ES supervised immunohistochemistry as a gynecologic pathologist and participated in immunohistochemistry analysis. UJ and HHH conceived the study and participated in its design and coordination and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SM reports research funding, advisory board participation, and honorary or travel expenses from AbbVie, AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Hubro, Medac, MSD, Novartis, Nykode, Olympus, PharmaMar, Pfizer, Roche, Sensor Kinesis, Teva, and Tesaro outside the submitted study. CD reports speaker fees from and participation in advisory boards at MSD, GSK, Tesaro, Clovis Oncology, and Roche outside the submitted study. All other authors declare no conflict of interest.
Ethical approval
This study is in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Patient data were anonymized. The study was approved by the ethics committee of the Ludwig-Maximilian-University Munich (reference number 19–261). During experimental and statistical analyses, the authors were blinded for clinicopathological parameters and survival data. The cancer tissue was no longer needed for clinical tests as histopathological diagnostics after surgery had been completed prior to this study.
Consent to participate
When the current study was performed, all diagnostic procedures were completed, and the patients’ data were anonymized. The ethical principles adopted in the Declaration of Helsinki 1975 have been respected. As per the declaration of our ethics committee, no written informed consent of the participants or permission to publish is needed given the circumstances described above. Researchers were blinded from patient data during experimental and statistical analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buchholz, A., Vattai, A., Fürst, S. et al. Prostaglandin E2 receptor EP1 expression in vulvar cancer. J Cancer Res Clin Oncol 149, 5369–5376 (2023). https://doi.org/10.1007/s00432-022-04487-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04487-z