Abstract
Purpose
Expression of the guanine nucleotide exchange factor epithelial cell transforming 2 (ECT2) is elevated in gastric cancer (GC) but its biological function in GC is poorly understood. MicroRNAs (miRNAs) have great potential as therapeutic targets for GC through their ability to modulate gene expression. In the present study, we sought to identify potential miRNA–mRNA–protein regulatory pathways that might control ECT2 expression and function in GC.
Methods
ECT2 expression was examined in clinical GC specimens by immunohistochemical staining, and protein levels were correlated with clinicopathological features and prognosis. TargetScan was used to identify potential ECT2 mRNA-complementary miRNAs, and the roles of ECT2 and miRNA-223-3p (miR-223-3p) in GC cell biology and signaling pathway activation were examined by targeted knockdown (KD) or overexpression (OE) of ECT2 and miR-223-3p in GC cell lines. A murine GC xenograft model was developed to explore the impact of ECT2 OE on tumor growth in vivo.
Results
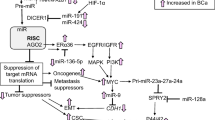
ECT2 expression was significantly elevated in GC specimens compared with normal gastric tissues and the level correlated positively with depth of invasion, ulceration, vascular tumor thrombus, neural invasion, and lymph node metastasis (p < 0.05). ECT2 was an independent prognostic factor for overall survival of GC patients (high ECT2 expression v.s. low ECT2 expression: χ2 = 29.831, p < 0.001). ECT2 KD or miR-223-3p OE markedly suppressed the proliferation, migration, and invasion of GC cells in vitro, whereas ECT2 OE had the opposite effects. ECT2 OE also promoted the growth of GC tumors in vivo. Tumor expression of Wnt2, β-catenin, and several downstream target proteins in GC cells were decreased by ECT2 KD or miR-223-3p OE but increased by ECT2 OE.
Conclusions
miR-223-3p regulates ECT2 expression to promote tumorigenic behavior of GC via activation of the Wnt/β-catenin signaling pathway, suggesting that ECT2 and miR-223-3p as potential therapeutic targets for GC.






Similar content being viewed by others
References
Bertuccio P, Chatenoud L, Levi F et al (2009) Recent patterns in gastric cancer:a global overview. Int J Cancer 125(3):666–673
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Chen J, Xia H, Zhang X et al (2015) ECT2 regulates the Rho/ERK signalling axis to promote early recurrence in human hepatocellular carcinoma. J Hepatol 62:1287–1295
Chen Y, Tian P, Liu Y (2017) P53 and protein phosphorylation regulate the oncogenic role of epithelial cell transforming 2 (ECT2). Med Sci Monit 23:3154–3160
Chen W, Sun K, Zheng R et al (2018) Cancer incidence and mortality in China, 2014. Chin J Cancer Res 30:1–12
Chen S, Zhu X, Zheng J, Xu T, Xu Y, Chen F (2021) miR-30a-5p regulates viability, migration, and invasion of lung adenocarcinoma cells via targeting ECT2. Comput Math Methods Med 2021:6241469
Fang G, Liu J, Wang Q et al (2017) MicroRNA-223–3p regulates ovarian cancer cell proliferation and invasion by targeting SOX11 expression. Int J Mol Sci 18:E1208
Fang ZQ, Li MC, Zhang YQ, Liu XG (2018) MiR-490-5p inhibits the metastasis of hepatocellular carcinoma by down-regulating E2F2 and ECT2. J Cell Biochem 119(10):8317–8324
Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H (2020) PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci 262:118513
Gong DY, Chen X, Yang TL et al (2020) Upregulation of ECT2 is associated with transcriptional program of cancer stem cells and predicts poor clinical outcome in gastric cancer. Oncol Lett 20(4):54
Guo J, Cao R, Yu X et al (2017) MicroRNA-223–3p inhibits human bladder cancer cell migration and invasion. Tumour Biol 39:1010428317691678
Guo Q, Xu J, Huang Z et al (2021) ADMA mediates gastric cancer cell migration and invasion via Wnt/β-catenin signaling pathway. Clin Transl Oncol 23(2):325–334
He B, Zhao Z, Cai Q et al (2020) miRNA-based biomarkers, therapies, and resistance in Cancer. Int J Biol Sci 16(14):2628–2647
Hill M, Tran N (2021) miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 14(4):dmm047662
Hu J, Shan Z, Hu K et al (2016) miRNA-223 inhibits epithelial-mesenchymal transition in gastric carcinoma cells via Sp1. Int J Oncol 49(1):325–335
Kang W, Tong JH, Chan AW et al (2012) Stathmin1 plays oncogenic role and is a target of microRNA-223 in gastric cancer. PLoS ONE 7(3):e33919
Kang HG, Kim WJ, Noh MG, Chun KH, Kim SJ (2020) SPON2 is upregulated through notch signaling pathway and promotes tumor progression in gastric cancer. Cancers (Basel) 12(6):1439
Leung WK, Bai AH, Chan VY, Yu J, Chan MW et al (2004) Effect of peroxisome proliferator activated receptor gamma ligands on growth and gene expression profiles of gastric cancer cells. Gut 53:331–338
Li PF, Chen SC, Xia T et al (2014) Non-coding RNAs and gastric cancer. World J Gastroenterol 20(18):5411–5419
Li H, Jia Y, Wang Y (2019) Targeting HIF-1α signaling pathway for gastric cancer treatment. Pharmazie 74(1):3–7
Liu B, Yang H, Taher L et al (2018) Identification of prognostic biomarkers by combined mRNA and miRNA expression microarray analysis in pancreatic cancer. Transl Oncol 11:700–714
Matthews HK, Delabre U, Rohn JL et al (2012) Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression. Dev Cell 23(2):371–83
Oksuz Z, Serin MS, Kaplan E et al (2015) Serum microRNAs; miR-30c-5p, miR-223-3p, miR-302c-3p and miR-17-5p could be used as novel non-invasive biomarkers for HCV-positive cirrhosis and hepatocellular carcinoma. Mol Biol Rep 42:713–720
Schmitt AM, Chang HY (2016) Long noncoding RNAs in Cancer pathways. Cancer Cell. 29:452–63
Sun XD, Mu R, Zhou YS et al (2004) Analysis of mortality rate of stomach cancer and its trend in twenty years in China. Clin Oncol 26(1):4–9
Sun BY, Wei QQ, Liu CX et al (2020) ECT2 promotes proliferation and metastasis of esophageal squamous cell carcinoma via the RhoA-ERK signaling pathway. Eur Rev Med Pharmacol Sci 24(15):7991–8000
Tatsumoto T, Xie X, Blumenthal R et al (1999) Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol 147(5):921–28
Togasaki K, Sugimoto S, Ohta Y et al (2021) Wnt signaling shapes the histologic variation in diffuse gastric cancer. Gastroenterology 160(3):823–830
Tutar Y (2014) miRNA and cancer; computational and experimental approaches. Curr Pharm Biotechnol 15(5):429
Wang XR, Tong ZS, Liu H (2019) MiR-223-3p targeting epithelial cell transforming sequence 2 oncogene inhibits the activity, apoptosis, invasion and migration of MDA-MB-468 breast cancer cells. Onco Targets Ther 12:7675–7684
Wang JX, Yang S, Min L et al (2021) ECT2 increases the stability of EGFR and tumorigenicity by inhibiting Grb2 ubiquitination in pancreatic cancer. Front Oncol 10:589241
Xu J, Yao Q, Hou Y et al (2013) miR-223/Ect2/p21 signaling regulates osteosarcoma cell cycle progression and proliferation. Biomed Pharmacother 67:381–386
Yang XZ, Cheng TT, He QJ et al (2018) LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer 17(1):126
Yu J, Cheng YY, Tao Q, Cheung KF, Lam CN et al (2009) Methylation of protocadherin 10, a novel tumor suppressor, is associated with poor prognosis in patients with gastric cancer. Gastroenterology 136:640–651
Zhang X, Wu J (2021) LINC00665 promotes cell proliferation, invasion, and metastasis by activating the TGF-β pathway in gastric cancer. Pathol Res Pract 224:153492
Zhang H, Geng Y, Sun C, Yu J (2021) Upregulation of ECT2 predicts adverse clinical outcomes and increases 5-fluorouracil resistance in gastric cancer patients. J Oncol 2021:2102890
Zhi T, Jiang K, Xu X et al (2019) ECT2/PSMD14/PTTG1 axis promotes the proliferation of glioma through stabilizing E2F1. Neuro Oncol 21(4):462–473
Zhou S, Wang P, Su X et al (2017) High ECT2 expression is an independent prognostic factor for poor overall survival and recurrence-free survival in non-small cell lung adenocarcinoma. PLoS One 12:e0187356
Acknowledgements
The authors acknowledge support from the Key Research Project of Wannan Medical College (Nos. WK2022ZF03, WK2021ZF18), and Wuhu City Science and Technology Project (No. 2021cg36, No. 2021cg45, No. 2021yf63).
Author information
Authors and Affiliations
Contributions
LL: study design, cell experiment and writing up of the work; PWL: animal experiment and data analysis; CYH: data collection and data analysis; CFX: study design and proofread the work. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Liu, P., He, C. et al. miRNA-223-3p regulates ECT2 to promote proliferation, invasion, and metastasis of gastric cancer through the Wnt/β-catenin signaling pathway. J Cancer Res Clin Oncol 149, 121–134 (2023). https://doi.org/10.1007/s00432-022-04453-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04453-9