Abstract
Background
Combination therapy with immune checkpoint inhibitors (ICIs) and chemotherapy (ICI + chemotherapy) has become the standard first line treatment for driver oncogene-negative advanced non-small-cell lung cancer (NSCLC). However, it may be more toxic compared to monotherapy, which limits its use. Moreover, the feasibility of the combination therapy in clinical practice remains unknown.
Methods
We conducted a cohort study to determine the implementation rate of ICI + chemotherapy in clinical practice. We retrospectively reviewed clinical data from advanced NSCLC patients who received systemic therapy at 13 institutions between December 2018 and December 2020.
Results
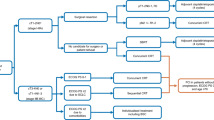
After excluding 154 patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) gene alterations, a total of 919 NSCLC patients were included. Among them, 442 were treated with ICI + chemotherapy (48%), whereas 477 were treated with other therapies (52%). Among these 477 patients, 340 did not receive ICI + chemotherapy because of intolerance (71%); thus, more than one-third of the advanced NSCLC patients do not benefit from the combination therapy due to intolerance. Among the 659 NSCLC patients for whom PD-L1 was < 50% or unknown, only 342 received the ICI + chemotherapy combination (52%) even though it is considered preferable to either therapy alone; the remaining 318 patients were treated with other therapies (48%). Among the 318 patients who did not receive ICI + chemotherapy, 274 were intolerant to it (86%).
Conclusion
Our results revealed that a substantial proportion of advanced NSCLC patients did not benefit from ICI + chemotherapy due to intolerance. As treatments for NSCLC are moving toward combinations for greater efficacy, their feasibility in clinical practice must be taken into consideration.



Similar content being viewed by others
References
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY-S, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC (2018) KEYNOTE-189 investigators, pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O’Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS (2019) Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381:2020–2031. https://doi.org/10.1056/NEJMOA1910231
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, Majem M, Fidler MJ, de Castro G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (london, England) 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM (2018) KEYNOTE-407 investigators, pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051. https://doi.org/10.1056/NEJMoa1810865
Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A, Sakai H, Lingua A, Salman P, Souquet P-J, De Marchi P, Martin C, Pérol M, Scherpereel A, Lu S, John T, Carbone DP, Meadows-Shropshire S, Agrawal S, Oukessou A, Yan J, Reck M (2021) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial., Lancet. Oncol 22:198–211. https://doi.org/10.1016/S1470-2045(20)30641-0
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
Conception and design: EI; data acquisition: CA, TY, KI, TT, KF, NO, HK, DK, KW, MI, NO, FO, HI, HK, and ST; data analysis and interpretation: EI, KH, YM and KK; writing and/or review of manuscript: all the authors.
Corresponding author
Ethics declarations
Conflict of interest
Eiki Ichihara received honoraria from AstraZeneca K.K., Novartis, Janssen Pharmaceutical K.K., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Pfizer, Bristol-Myers Squibb Co. Ltd., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., and Boehringer Ingelheim. Eiki Ichihara received research funding from Janssen Pharmaceutical K.K., Pfizer, Bristol-Myers Squibb Co. Ltd., Ono Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Co. Ltd. Toshihide Yokoyama received honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., Pfizer Japan Inc, Bristol-Myers Squibb Co. Ltd., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Nippon Kayaku Co., Ltd., Boehringer Ingelheim Japan Inc. Toshihide Yokoyama also recieved research funding from MSD, Takeda Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Bristol-Myers Squibb Co. Ltd., Boehringer Ingelheim Japan Inc. Koji Inoue received honoraria from AstraZeneca, Takeda, Shionogi, Kyowa Kirin, Ono, Towa, Chugai and Boehringer ingelheim. Tomoki Tamura received honoraria from Chugai Pharmaceutical Co., Ltd, and Taiho Pharmaceutical Co., Ltd. Keiichi Fujiwara received honoraria from MSD, AstraZeneca, Ono Pharmaceutical Co., Sanofi, and Eli Lilly Japan. Hirohisa Kano received honoraria from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb and ONO Pharmaceutical. Masaaki Inoue received honoraria from Bristol Myers Squibb, Chugai Pharmaceutical Co, Daiichi-Sankyo, Kyowa Kirin Co, AstraZeneca and Taiho Pharmaceutical Co. Hiroshi Kobe received honoraria from Boehringer ingelheim and Chugai pharmaceutical. Katsuyuki Hotta received honoraria from MSD, AstraZeneca, Chugai pharmaceutical, Lilly, BMS and Abbvie. He also received research funding from Pfizer, AstarZeneca, Chugai pharmaceutical, Lilly, Takeda Pharmaceutical, MSD, BMS, Ono pharmaceutical, Taiho pharmaceutical and Boehringer ingelheim. Yoshinobu Maeda received honoraria from AstraZeneca K.K., Amgen K.K., ONO pharmaceutical co., LTD., Bristol-Myers Squibb K.K., Takeda Pharmaceutical ltd., Chugai Pharmaceutical Co.,Ltd., Novartis, Pfizer Japan Inc., and Janssen Pharmaceutical K.K. Yoshinobu Maeda also received reserch funding from Chugai Pharmaceutical Co.,Ltd., AstraZeneca K.K., Novartis and Janssen Pharmaceutical K.K. Katsuyuki Kiura received honoraria from AstraZeneca, Novartis, Lilly, Taiho Pharmaceutical Co. td., Chugai Pharmaceutical, Pfizer, Ono pharmaceutical, MSD, BMS, Boehringer ingelheim, Daiichi Sankyo, Takeda and Nippon Kayaku. He also received research funding from Merk BioPharma, Kyowa Kirin, Pfizer, MSD, Boehringer ingelheim, Shionogi pharmaceutical, and Ono pharmaceutical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2022_4415_MOESM2_ESM.doc
Supplementary file2 Table S1. Details of intolerance in all the investigated patients. Table S2. Details of intolerance in PD-L1<1% or unknown NSCLC patients. Table S3. Details of intolerance in PD-L1≥50% NSCLC patients (DOC 50 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ando, C., Ichihara, E., Yokoyama, T. et al. More than one-third of advanced non-small-cell lung cancer patients do not receive immunochemotherapy due to intolerance. J Cancer Res Clin Oncol 149, 4933–4938 (2023). https://doi.org/10.1007/s00432-022-04415-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04415-1