Abstract
Purpose
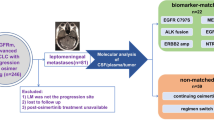
Leptomeningeal metastasis (LM) is a serious complication of non-small cell lung cancer (NSCLC), particularly in patients with EGFR mutations. In this study, we investigated the survival outcomes of patients with EGFR-mutant NSCLC who have developed LM and explored the factors associated with their survival.
Methods
From April 2018 to November 2021, patients with EGFR-mutant NSCLC who underwent cerebrospinal fluid (CSF) sampling under the clinical suspicion of LM were enrolled. The patients’ clinicodemographic characteristics, treatment history including whole-brain radiation therapy (WBRT), overall survival (OS), and intracranial progression-free survival (icPFS) were measured. EGFR mutations in cell-free tumor DNA (ctDNA) of CSF, including T790M mutation, were analyzed.
Results
We enrolled 62 patients with NSCLC. The median time form diagnosis to LM was 23.1 months and 16 (25.8%) patients had history of prior third-generation EGFR-TKI use. EGFR mutation in CSF ctDNA was detected in 53 patients (85.5%); of them, 10 (16.1%) had T790M mutation. The patients’ icPFS and OS after osimertinib were 6.43 and 9.37 months, respectively, and were comparable among patients with different sensitive EGFR mutations, indicating that EGFR mutation status did not affect osimertinib efficacy. Patients who received WBRT after LM had numerically higher icPFS and OS compared to those without. Multivariate analysis revealed that lack of prior exposure to third-generation EGFR-TKI was associated with better OS.
Conclusions
Osimertinib is effective in patients with EGFR-mutant NSCLC who developed LM and prior third-generation EGFR-TKI use was associated with poor survival in these patients. The role of WBRT warrants further investigation.



Similar content being viewed by others
References
Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, Greystoke A, Mok T (2020) Osimertinib for patients With leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol 15(4):637–648. https://doi.org/10.1016/j.jtho.2019.12.113
Boire A, Brandsma D, Brastianos PK, Le Rhun E, Ahluwalia M, Junck L, Soffietti R (2019) Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol 21(5):571–584. https://doi.org/10.1093/neuonc/noz012
Chamberlain M, Junck L, Brandsma D, Soffietti R, Rudà R, Raizer J, Jaeckle KA (2017) Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol 19(4):484–492. https://doi.org/10.1093/neuonc/now183
Cheng H, Perez-Soler R (2018) Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 19(1):e43–e55. https://doi.org/10.1016/s1470-2045(17)30689-7
Chiang CL, Huang HC, Shen CI, Luo YH, Chen YM, Chiu CH (2020) Post-progression survival in secondary EGFR T790M-mutated non-small-cell lung cancer patients with and without osimertinib after failure of a previous EGFR TKI. Target Oncol 15(4):503–512. https://doi.org/10.1007/s11523-020-00737-7
Chiang CL, Huang HC, Luo YH, Chiu CH (2021a) Cerebrospinal fluid as a medium of liquid biopsy in the management of patients with non-small-cell lung cancer having central nervous system metastasis. Front Biosci (landmark Ed) 26(12):1679–1688. https://doi.org/10.52586/5060
Chiang CL, Lee CC, Huang HC, Wu CH, Yeh YC, Shen CI, Chiu CH (2021b) Utility of cerebrospinal fluid cell-free DNA in patients with EGFR-mutant non-small-cell lung cancer with leptomeningeal metastasis. Target Oncol 16(2):207–214. https://doi.org/10.1007/s11523-021-00791-9
Fan C, Zhao Q, Li L, Shen W, Du Y, Teng C, Xin T (2021) Efficacy and safety of intrathecal pemetrexed combined with dexamethasone for treating tyrosine kinase inhibitor-failed leptomeningeal metastases from EGFR-mutant NSCLC-a prospective, open-label, single-arm phase 1/2 clinical trial (unique identifier: ChiCTR1800016615). J Thorac Oncol 16(8):1359–1368. https://doi.org/10.1016/j.jtho.2021.04.018
How J, Mann J, Laczniak AN, Baggstrom MQ (2017) Pulsatile erlotinib in EGFR-positive non-small-cell lung cancer patients with leptomeningeal and brain metastases: review of the literature. Clin Lung Cancer 18(4):354–363. https://doi.org/10.1016/j.cllc.2017.01.013
Jenkins S, Yang JC, Jänne PA, Thress KS, Yu K, Hodge R, Shepherd FA (2017) EGFR mutation analysis for prospective patient selection in two phase II registration studies of osimertinib. J Thorac Oncol 12(8):1247–1256. https://doi.org/10.1016/j.jtho.2017.05.002
John T, Akamatsu H, Delmonte A, Su WC, Lee JS, Chang GC, Wu YL (2018) EGFR mutation analysis for prospective patient selection in AURA3 phase III trial of osimertinib versus platinum-pemetrexed in patients with EGFR T790M-positive advanced non-small-cell lung cancer. Lung Cancer 126:133–138. https://doi.org/10.1016/j.lungcan.2018.10.027
Kuiper JL, Hendriks LE, van der Wekken AJ, de Langen AJ, Bahce I, Thunnissen E, Smit EF (2015) Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer 89(3):255–261. https://doi.org/10.1016/j.lungcan.2015.05.023
Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, Preusser M (2017) EANO-ESMO clinical practice guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 28(suppl_4):iv84–iv99. https://doi.org/10.1093/annonc/mdx221
Lee J, Choi Y, Han J, Park S, Jung HA, Su JM, Ahn MJ (2020) Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol 15(11):1758–1766. https://doi.org/10.1016/j.jtho.2020.06.018
Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, Wu YL (2016) Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol 11(11):1962–1969. https://doi.org/10.1016/j.jtho.2016.06.029
Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang Z, Ye JY, Wu YL (2018) Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol 29(4):945–952. https://doi.org/10.1093/annonc/mdy009
Li C, Nie W, Guo J, Xiong A, Zhong H, Chu T, Zhang X (2021) Osimertinib alone as second-line treatment for brain metastases (BM) control may be more limited than for non-BM in advanced NSCLC patients with an acquired EGFR T790M mutation. Respir Res 22(1):145. https://doi.org/10.1186/s12931-021-01741-9
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Papadimitrakopoulou VA (2017) Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 376(7):629–640. https://doi.org/10.1056/NEJMoa1612674
Nanjo S, Hata A, Okuda C, Kaji R, Okada H, Tamura D, Katakami N (2018) Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer 118(1):32–37. https://doi.org/10.1038/bjc.2017.394
Park S, Lee MH, Seong M, Kim ST, Kang JH, Cho BC, Ahn MJ (2020) A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol 31(10):1397–1404. https://doi.org/10.1016/j.annonc.2020.06.017
Remon J, Le Rhun E, Besse B (2017) Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev 53:128–137. https://doi.org/10.1016/j.ctrv.2016.12.006
Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, Vansteenkiste J (2018) CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. https://doi.org/10.1200/jco.2018.78.3118
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Ramalingam SS (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378(2):113–125. https://doi.org/10.1056/NEJMoa1713137
Suryavanshi M, Jaipuria J, Panigrahi MK, Goyal N, Singal R, Mehta A, Talwar V (2020) CSF cell-free DNA EGFR testing using DdPCR holds promise over conventional modalities for diagnosing leptomeningeal involvement in patients with non-small cell lung cancer. Lung Cancer 148:33–39. https://doi.org/10.1016/j.lungcan.2020.07.034
Thomas NJ, Myall NJ, Sun F, Patil T, Mushtaq R, Yu C, McCoach CE (2022) Brain metastases in EGFR- and ALK-positive NSCLC: outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J Thorac Oncol 17(1):116–129. https://doi.org/10.1016/j.jtho.2021.08.009
Xie L, Nagpal S, Wakelee HA, Li G, Soltys SG, Neal JW (2019) Osimertinib for EGFR-mutant lung cancer with brain metastases: results from a single-center retrospective study. Oncologist 24(6):836–843. https://doi.org/10.1634/theoncologist.2018-0264
Xu H, Chen H, Kong J, Zhang Y, Liu S, Yang G, Wang Y (2021) Osimertinib for the treatment of epidermal growth factor receptor-mutated non-small cell lung cancer patients with leptomeningeal metastases and different T790M status. Ann Transl Med 9(11):937. https://doi.org/10.21037/atm-21-1249
Yan W, Jing W, An N, Tian Y, Guo D, Kong L, Yu J (2019a) The clinical characteristic and prognostic factors of leptomeningeal metastasis in patients with non-small-cell lung cancer-a retrospective study from one single cancer institute. Cancer Med 8(6):2769–2776. https://doi.org/10.1002/cam4.2156
Yan W, Liu Y, Li J, Han A, Kong L, Yu J, Zhu H (2019b) Whole brain radiation therapy does not improve the overall survival of EGFR-mutant NSCLC patients with leptomeningeal metastasis. Radiat Oncol 14(1):168. https://doi.org/10.1186/s13014-019-1376-z
Yu F, Ni J, Zeng W, Zhou Y, Guo T, Zeng Y, Zhu Z (2021) Clinical value of upfront cranial radiation therapy in osimertinib-treated epidermal growth factor receptor-mutant non-small cell lung cancer with brain metastases. Int J Radiat Oncol Biol Phys 111(3):804–815. https://doi.org/10.1016/j.ijrobp.2021.05.125
Zeng YD, Liao H, Qin T, Zhang L, Wei WD, Liang JZ, Chen LK (2015) Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget 6(10):8366–8376. https://doi.org/10.18632/oncotarget.3187
Zheng MM, Li YS, Tu HY, Jiang BY, Yang JJ, Zhou Q, Wu YL (2021) Genotyping of cerebrospinal fluid associated with osimertinib response and resistance for leptomeningeal metastases in EGFR-mutated NSCLC. J Thorac Oncol 16(2):250–258. https://doi.org/10.1016/j.jtho.2020.10.008
Funding
This work was supported by the Taipei Veterans General Hospital, Taiwan [grant number: V110B-008].
Author information
Authors and Affiliations
Contributions
Conceptualisation: CLC, YMC, and CHC. Data collection, experiment, and analysis: CLC, HLH, YCY, CCL, HCH, CIS, YHL, and CHC. Statistics: CLC and YCY. First draft preparation: CLC and TYC. Review and final approval: all the authors.
Corresponding authors
Ethics declarations
Conflict of interest
CLC had received honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, and Roche. YHL had received honoraria from AstraZeneca, Boehringer Ingelheim, and Pfizer. YMC had received honoraria from Boehringer Ingelheim, Eli Lilly, Roche/Genentech/Chugai, MSD, Pfizer, Novartis, BMS, Ono Pharmaceutical, AstraZeneca, and Takeda Oncology; and served as advisor for Boehringer Ingelheim, Eli Lilly, Roche/Chugai, MSD, AstraZeneca, and Takeda Oncology. CHC had received honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Janssen, Merck KGaA, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, Pfizer, Roche, and Takeda. Others declare no conflict of interest that might be relevant to the contents of this manuscript.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Taipei Veterans General Hospital, Taiwan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chiang, CL., Ho, HL., Yeh, YC. et al. Prognosticators of osimertinib treatment outcomes in patients with EGFR-mutant non-small cell lung cancer and leptomeningeal metastasis. J Cancer Res Clin Oncol 149, 5–14 (2023). https://doi.org/10.1007/s00432-022-04396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04396-1