Abstract
Background
The formation of neutrophil extracellular traps (NETs) was initially discovered as a novel immune response against pathogens. Recent studies have also suggested that NETs play an important role in tumor progression. This review summarizes the cellular mechanisms by which NETs promote distant metastasis and discusses the possible clinical applications targeting NETs.
Method
The relevant literature from PubMed and Google Scholar (2001–2021) have been reviewed for this article.
Results
The presence of NETs has been detected in various primary tumors and metastatic sites. NET-associated interactions have been observed throughout the different stages of metastasis, including initial tumor cell detachment, intravasation and extravasation, the survival of circulating tumor cells, the settlement and the growth of metastatic tumor cells. Several in vitro and in vivo studies proved that inhibiting NET formation resulted in anti-cancer effects. The biosafety and efficacy of some NET inhibitors have also been demonstrated in early phase clinical trials.
Conclusions
Considering the role of NETs in tumor progression, NETs could be a promising diagnostic and therapeutic target for cancer management. However, current evidence is mostly derived from experimental models and as such more clinical studies are still needed to verify the clinical significance of NETs in oncological settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neutrophil extracellular traps (NETs) are web-like chromatin structures that are made up of a DNA backbone decorated with histones and granule proteins. They are released from neutrophils and were initially described by Brinkmann et al. in 2004 (Brinkmann et al. 2004). They reported NET formation as an innate immune response, which can trap and kill bacteria (Brinkmann et al. 2004). Since the discovery of NETs, more details of NETs have been uncovered. A growing body of evidence shows that NET formation occurs not only in infectious disease, but can also be triggered by other various stimuli (Table 1) and NETs play instrumental roles in various non-infectious conditions, including malignancy (Albrengues et al. 2018; Boone et al. 2015; Cools-Lartigue et al. 2013; Demers et al. 2016; Martins-Cardoso et al. 2020; Miller-Ocuin et al. 2019; Monti et al. 2018; Najmeh et al. 2017; Nie et al. 2019; Park et al. 2016; Rayes et al. 2019; Ren et al. 2021; Teijeira et al. 2020; Tohme et al. 2016; Xiao et al. 2021; Yang et al. 2020a, b; Yazdani et al. 2019; Zha et al. 2020), autoimmune disease (Apel et al. 2021; Carmona-Rivera et al. 2015; Kahlenberg et al. 2013; Parackova et al. 2020; Schauer et al. 2014), vascular disease (Binet et al. 2020; Grässle et al. 2014; Kang et al. 2020; Quillard et al. 2015; Warnatsch et al. 2015), surgical stress and traumatic injury (Cools-Lartigue et al. 2013; Liu et al. 2019; Ren et al. 2021; Tohme et al. 2016; von Meijenfeldt et al. 2018). Lytic NETosis and non-lytic NETosis are terms used to describe two distinct processes of NET formation (Pieterse et al. 2016). During lytic NETosis, the plasma membranes of neutrophils break down resulting in the release of NETs and subsequent cell death (Fuchs et al. 2007). In contrast, plasma membranes remain intact during non-lytic NETosis, in which neutrophils export NETs by releasing vesicles that contain DNA (Thiam et al. 2020). In this review, we will discuss the cellular mechanisms of NETosis and highlight NET-mediated actions during metastasis.
The mechanisms of NET formation
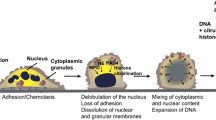
Lytic NETosis (Fig. 1a), also known as suicidal NET formation, is a special programmed cell death driven by reactive oxygen species (ROS). Various external stimuli, including crossing-linking of immune complex and Fcγ receptor (Alemán et al. 2016a, 2016b), interaction between interleukin-8 (IL-8) and C–X–C motif chemokine receptor 2 (CXCR2) (An et al. 2019; Nie et al. 2019; Zha et al. 2020), or direct stimulation by phorbol 12-myristate 13-acetate (PMA), are able to activate protein kinase C and lead to NADPH oxidase-dependent ROS production via the Raf-MEK-ERK signaling pathway (Gray et al. 2013; Hakkim et al. 2011). In some cases, ROS production may be NADPH oxidase-independent, for example, calcium ionophore-induced NETosis is mediated by calcium-dependent SK3 channel and relies on mitochondrial ROS production (Douda et al. 2015). The accumulation of intracellular ROS eventually leads to the rupture of azurophilic granules and the release of myeloperoxidase (MPO) and neutrophil elastase (NE). Myeloperoxidase and NE act synergistically to enhance their proteolytic activities and translocation into the nucleus (Branzk et al. 2014; Metzler et al. 2014; Papayannopoulos et al. 2010). Before reaching the nucleus, NE may cause degradation of the actin cytoskeleton, which leads to neutrophil immobilization and settlement of NETs within the foci of disease (Metzler et al. 2014). Once within the nucleus, NE could cleave histone H4 and unwind condensed chromatin. Besides NE-mediated histone modification, the citrullination of histones by peptidylarginine deiminase 4 (PAD4) is another mechanism that induces chromatin decompaction. Under the influence of Ca2+ and ROS (Rohrbach et al. 2012), PAD4 is activated and drives the conversion of arginine residues to citrullines in histone H3 and H4, which reduces the histones’ electrostatic attraction to DNA strands and promotes chromatin decondensation (Kaplan and Radic 2012). Chromatin swelling and nuclear lamin disassembly promotes the release of DNA into the cytoplasm (Li et al. 2020; Neubert et al. 2018), where cytoplasmic proteins could bind to DNA strands before the extrusion of NETs (Papayannopoulos et al. 2010). Gasdermin D (GSDMD), a pore-forming protein, which is activated by NE and caspase-4, mediates the final step of NETosis by inducing the expulsion of DNA into the extracellular space. Activated GSDMD not only forms pores in the plasma membrane, but also forms pores in the granule membrane and results in the release of more NE, which significantly enhances NET formation by establishing a positive-feedback loop (Sollberger et al. 2018).
Mechanisms of lytic and non-lytic NETosis. a Mechanism of lytic NETosis. External stimuli, such as Phorbol 12-myristate 13-acetate (PMA), IL-8 and immune complex, could activate membrane-bound NADPH oxidase (NOX) to produce reactive oxygen species (ROS). Accumulation of ROS leads to the release of neutrophil elastase (NE) and myeloperoxidase (MPO) from azurosomes. NE and peptidylarginine deiminase 4 (PAD4) both contribute to histone modification and chromatin decompaction, which are followed by nuclear membrane breakage. Chromatin and nuclear proteins are then released into cytoplasmic space, where they interact with cytoplasmic proteolytic enzymes and antibacterial peptides. Gasdermin D (GSDMD) induces plasma membrane rupture and mediates the expulsion of NETs into the extracellular space. b Mechanism of non-lytic NETosis. Lipopolysaccharide (LPS) or HMGB1 could induce neutrophils to release NETs in a non-lytic manner through TLR2/4-mediated signaling pathways. Alternatively, TLR4-activated platelets could also predispose non-lytic NETosis through the interaction between P-selectin and its ligand, PSGL-1. Unlike lytic NETosis, non-lytic NETosis is ROS-independent and DNA-NETs are released via vesicular export without the rupture of plasma membrane
In 2007, Clark et al. reported that interactions between platelets and neutrophils could induce NETosis without resulting in neutrophil death (Clark et al. 2007). When neutrophils release NETs under the stimulation of platelets, their plasma membrane integrity was maintained (shown by lack of staining by cell-impermeant nuclear dye Sytox Green). Later this process was named as non-lytic NETosis or vital NETosis (Fig. 1b). Most host-defense functions, such as phagocytosis, remain intact in neutrophils undergoing non-lytic NETosis (Yipp et al. 2012). Unlike lytic NETosis, which usually takes several hours to occur, non-lytic NETosis happens much faster. It has been documented that several pathogens, including S. aureus and C. albicans, could promote vital NETosis by activating TLR2 or C3a receptors (Yipp et al. 2012). Additionally, the crosstalk between neutrophils and platelets, which are activated by the LPS-TLR4 axis, also contributes to non-lytic NETosis (Clark et al. 2007; Pieterse et al. 2016). Although the molecular mechanism of non-lytic NETosis remains unclear, Pilsczek et al. have pointed out that DPI, a NADPH oxidase blocker, failed to inhibit S. aureus-induced NETosis over the first hour, which suggests that non-lytic NETosis may be NOX-independent (Pilsczek et al. 2010).
Due to the diversity of upstream signaling pathways, the same stimulant may result in different types of NETosis. For example, LPS derived from P. aeruginosa and E. coli O128:B12 often induces ROS-dependent lytic NETosis (Pieterse et al. 2016). However, when neutrophils are co-cultured with platelets, LPS released by P. aeruginosa and E. coli O111:B4 is more likely to induce vital NETosis (Pieterse et al. 2016). The type of NETosis may also change over time. When neutrophils are exposed to S. aureus, non-lytic NETosis predominates in the early phase, but is replaced by lytic NETosis in the late stage. The content of non-lytic and lytic NETs may differ depending on the time of release and cell membrane integrity. Despite both mechanisms having bactericidal effects, non-lytic NETs have significantly lower protease content than lytic NETs (Pieterse et al. 2016; Pilsczek et al. 2010). NET-bound proteases, such as NE and MPO, are potent inducers of several NET-mediated responses (Albrengues et al. 2018; Chen et al. 2021; Yazdani et al. 2019). These two difference types of NETosis may serve different functions, the rapid release of NETs without neutrophil destruction could allow timely neutralization of pathogens. Whereas the slow release of NETs in a lytic manner, containing more bioactive compounds, may lead to or exacerbate inflammation and influence the progression of chronic disease.
The functional components of NETs and their mechanisms of action
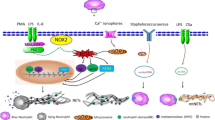
In addition to their DNA backbone, NETs could have more than one hundred possible proteins as their constituents (Lim et al. 2018; Urban et al. 2009). Based on their origin and function, the most commonly found NET proteins can be classified into the following categories: nuclear proteins (e.g. citrullinated histone, HMGB1), antimicrobial and pro-inflammatory peptides (e.g. cathelicidin, calprotectin, lactotransferrin, complement C3), cytoskeletal elements (e.g. cytokeratin, actin), proteolytic enzymes (e.g. NE, MMP, cathepsin G, lipocalin-2), and other metabolic enzymes (e.g. transketolase, enolase). The interactions between DNA and bound proteins largely amplify the bioactivities of NETs. Extracellular DNA of NETs could protect bound proteins from denaturation, preserve the biological functions of proteins (Hahn et al. 2019; Papayannopoulos et al. 2010; Saffarzadeh et al. 2012) and serve as a proteolysis scaffold (Albrengues et al. 2018). The NET-bound proteins in turn protect DNA from nuclease degradation (Neumann et al. 2014) and enhance DNA recognition (Apel et al. 2021; Garcia-Romo et al. 2011). Because of the diversity of components, NETs can be recognized by a variety of cellular receptors which lead to the activation of different signaling pathways. The receptors for NET components and their biological actions are summarized in Table 2.
Pattern recognition receptors (PRRs) are a class of host sensors that recognize both exogenous pathogen-associated molecular patterns (PAMPs) and endogenous damage-associated patterns (DAMPs). NETs are deemed as DAMPs and are recognized by the vast majority of pattern recognition receptors (PRRs). Toll-like receptors (TLRs), a family of PRRs widely expressed on innate immune cells and non-immune cells, are extensively studied in NET-mediated response. Several proteins on NETs, such as NE and histones, can activate plasma membrane-located TLR2 and TLR4. Once NETs are taken up by target cells, the cytoplasmic receptor TLR9 can recognize CpG motifs of DNA-NETs. Activation of TLRs and their downstream MyD88-dependent signaling pathways then induce pro-inflammatory changes in target cells, which exacerbates disease progression. Exposure to the DNA-peptide complex can provoke intracellular signaling more easily than exposure to DNA alone with the best example being cathelicidin on NETs. In the context of autoimmune diseases, such as psoriasis and SLE, the combination of cathelicidin and DNA enables inert DNA to become a potent TLR9 activator that induces cytokine production in plasmacytoid dendritic cells (Garcia-Romo et al. 2011; Lande et al. 2011, 2007). Similar NET-induced changes were noted in macrophages, in which cathelicidin-NET complexes activate NLPR3 inflammasomes and lead to the release of IL-1β and IL-18 (Kahlenberg et al. 2013; Warnatsch et al. 2015). Secreted IL-18 could further promote NETosis and amplify the inflammation response.
The receptor for advanced glycation end products (RAGE) is another transmembrane receptor which serves as PRRs (Sparvero et al. 2009). Several constituents of NETs, such as HMGB1 and S100 proteins, are considered RAGE ligands (Sparvero et al. 2009). RAGE plays an important role in both NET formation and NET-mediated response (Boone et al. 2015; Miller-Ocuin et al. 2019). HMGB1 on NETs was reported to activate RAGE and trigger downstream NF-κB signaling in glioblastoma (Zha et al. 2020), while DNA-NETs were found to be responsible for RAGE-dependent activation of pancreatic stellate cells (Miller-Ocuin et al. 2019).
Similar to TLR9, cyclic GMP-AMP synthase (cGAS) is intracellular DNA sensor which can also recognize phagocytosed NETs. The activation of NETs-mediated cGAS-STING pathways prime macrophages to type I interferon production and contribute to the pathogenesis of several diseases (Apel et al. 2021; Kang et al. 2020). Besides PRRs and cGAS, NETs can also interact with CDCC25, a transmembrane protein, which was poorly understood in the past. It was recently discovered that through stimulation with DNA-NETs, CCDC25 on tumor cells could initiate the ILK-β parvin signaling pathway to remodel the intracellular cytoskeleton, which facilitates migration and adhesion of tumor cells (Yang et al. 2020a).
Aside from binding to cellular receptors, NETs also take advantage of attached proteases to elicit cellular response. As examples, NET-bound elastase can prevent macrophages from efferocytosis of apoptotic cells by disruption of αvβ3/αvβ5 integrins (Chen et al. 2021). NET-bound cathepsin G could process pro-IL-1α to IL-1α, a powerful inducer of adhesion molecules and tissue factors on endothelial cells (Folco et al. 2018).
NETs and cancer progression
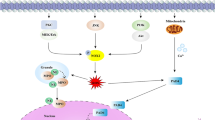
There is increasing evidence that tumor-derived substances, including IL-8 and HMGB1, could induce NET formation (Nie et al. 2019; Ren et al. 2021; Zha et al. 2020). NETs eventually contribute to the tumor growth and distant metastasis (Fig. 2). The presence of intra-tumoral NETs were confirmed by immunofluorescence staining in multiple cancer tissues from mice and humans (Guan et al. 2021; Oklu et al. 2017; Park et al. 2016; Yang et al. 2020a, b). Furthermore, markers of circulating NETs, such as H3Cit and MPO-dsDNA, are found to be elevated in patients with lung metastases (Yang et al. 2020a) and colorectal liver metastases (Tohme et al. 2016). To some extent, the circulating NETs may reflect the severity of the malignant disease. In HCC patients, H3Cit-DNA level was significantly higher in HCC patients with tumors ≥ 8 cm (Zenlander et al. 2021). A similar study showed that the level of MPO-dsDNA was much higher in patients with advanced stage esophagogastric or lung adenocarcinoma than those with low stage carcinoma (Rayes et al. 2019).
NETs promote tumor growth and distant metastasis. In primary tumor, the release of IL-8 and HMGB1 predisposes NET formation, which induces epithelial–mesenchymal transition (EMT) and promote tumor cell proliferation, migration, and invasion of tumor cells. NETs also contribute to local endothelial–mesenchymal transition and endothelial dysfunction, eventually facilitating the intravasation of tumor cells. During dissemination, NETs may protect circulating tumor cells (CTCs) from cytotoxic attacks of immune cells. NETs may also activate platelets and provoke a procoagulant state. Infection and inflammation could induce the release of NETs in the distant organs, leading to potential upregulation in the expression of endothelial adhesion molecules. Once CTCs arrive the host organ, the pre-existing NET formation could mediate the adhesion and settlement of CTCs. Apart from directly promoting tumor cell proliferation, NETs could induce metastatic tumor growth by recruiting tumor stromal cells such as cancer-associated fibroblasts (CAFs). Furthermore, NETs could modify the extracellular matrix (ECM) to enhance distant metastasis. For instance, NET-remodeled laminin awakens dormant tumor cells, while degradation of thrombospondin-1 (TSP-1) minimizes its inhibitory effects on tumorigenesis
The occurrence of NETosis may precede the arrival of circulating tumor cells. NETs are detected in various conditions other than malignancies, such as local infection, chronic obstructive pulmonary disease (Dicker et al. 2018), steatohepatitis (van der Windt et al. 2018), liver cirrhosis (Zenlander et al. 2021) and surgical stress (Cools-Lartigue et al. 2013; Ren et al. 2021; von Meijenfeldt et al. 2018). The pre-existing NETs may shape a pre-metastatic niche to host the arriving tumor cells, for example, liver ischemia reperfusion (I/R) injury models demonstrated that surgical stress could result in widespread NETosis (Zhang et al. 2020a), NETs deposited in the lung microvasculature could capture circulating tumor cells and promote distant metastasis (Ren et al. 2021).
NETs promote the epithelial–mesenchymal transition of cancer cells
Epithelial–mesenchymal transition (EMT) is a cellular programme involved in embryogenesis, tissue regeneration, wound healing, and metastasis. Key features of EMT include the disruption of cell–cell and cell–matrix adhesion, the loss of cellular polarity, and cytoskeletal remodeling (Dongre and Weinberg 2019). This transition is adopted by tumor cells to enable migration and invasion. Recent studies demonstrated that NETs and their components are able to induce EMT in various types of tumor cells, including breast cancer cells (Martins-Cardoso et al. 2020), gastric cancer cells (Zhu et al. 2021) and pancreatic cancer cells (Jin et al. 2021; Kajioka et al. 2021). After exposure to NETs in vitro, tumor cells developed morphology alterations, which are characterized by elongated fibroblast-like shape and the loss of adhesion to the flask wall. Immunoblotting showed reduced expression of epithelial markers, such as E-cadherin, and enhanced expression of mesenchymal markers, such as N‐cadherin, vimentin and α-SMA (Kajioka et al. 2021; Zhu et al. 2021). The mRNA levels of EMT regulators, including ZEB, SLUG and SNAIL, are also upregulated in NET-treated tumor cells. These transcriptional and translational alterations observed in NET-induced EMT are similar to the changes in TGF-β-induced EMT (Katsuno et al. 2013). NET-induced EMT and related tumor progression could be inhibited in vivo by administration of NET inhibitors, DNase and PAD4 blocker (Zhu et al. 2021). Nevertheless, the exact mechanism behind NET-induced EMT are still unclear. Kajioka et al. believed that NET-bound HMGB1 is the main activator of NET-induced EMT. Similar findings were observed when pancreatic tumor cells were treated with free HMGB1 and NET-induced EMT was blocked when anti-HMGB1 thrombomodulin was applied (Kajioka et al. 2021). However, Jin et al. and Martins-Cardoso et al. suggested that NET-mediated pro-inflammatory response and upregulation of inflammatory cytokines are responsible for EMT changes (Jin et al. 2021; Martins-Cardoso et al. 2020). It is worth noting that NET-induced EMT is not only found in cancerous conditions. In vitro airway models and lung biopsies of deceased patients suggest that NETs may also contribute to the EMT of alveolar epithelial cells and lung fibrosis in COVID-19 (Pandolfi et al. 2021).
NETs may facilitate the intravasation and extravasation of cancer cells
Metastasis requires the entry of tumor cells into the circulation (intravasation) and their exit from the circulation (extravasation) into host tissue. Multiple strategies are adopted by tumor cells to cross the endothelial barrier during these processes. The EMT described above allows tumor cells to abrogate cell–cell adhesion and pass through the endothelial junctions in an advantageous shape. In addition to promoting epithelial–mesenchymal transition in tumor cells, NETs also contribute to endothelial-to-mesenchymal transition. VE-cadherin/β-catenin is an adherens junction complex which maintains the integrity of the endothelial barrier. Degradation of cadherin by NET-bound elastase breaks down the adhesion between endothelial cells. Undocked intracellular β-catenin then translocates into the nucleus and subsequently activates the EMT-associated signaling pathway. Treatment with NETs significantly increases vascular leakage and induce proteinuria in vivo (Pieterse et al. 2017). Similar studies have shown that NETs disrupt the integrity of the blood–brain barrier during stroke (Kang et al. 2020), resulting in increased permeability and delayed vascular damage. The endothelial barrier is sustained by endothelial cells and adhesion molecules; injury of endothelial cells leads to barrier dysfunction and facilitates trans-endothelial migration of tumor cells. One previous study reported that tumor cells can promote trans-endothelial migration by inducing endothelial necroptosis (Strilic et al. 2016). In fact, several studies have confirmed that NETs directly contribute to endothelial cell apoptosis (Binet et al. 2020; Gupta et al. 2010; Saffarzadeh et al. 2012), which can cause a breach that may provide a gateway for tumor cell passage. NETs contain several collagenases, such as MMP2 and MMP9, which might further decrease cell adherence and increase damage to the endothelial barrier by degrading basement membrane proteins (Quillard et al. 2015). In addition, NETs may enhance the motility of tumor cells by inducing cytoskeleton rearrangement via the CCDC25-ILK signaling pathway (Yang et al. 2020a), which may further facilitate tumor cell transmigration across the endothelium.
A potential role of NETs in inducing a procoagulant state and escorting circulating tumor cells to the metastatic niche
When tumor cells leave the primary site of malignancy to circulate in blood and lymph, they are at high risk of being killed by cytotoxic immune cells and the shear stress generated by blood flow (Regmi et al. 2017). To increase the chance of survival, circulating tumor cells (CTCs) either travel as cell clusters (Aceto et al. 2014) or recruit platelets and fibrins to form tumor cell-platelet aggregates (Anvari et al. 2021; Egan et al. 2014; Heeke et al. 2019). The attached platelets and fibrins not only provide an immune cell-resistant physical shield (Palumbo et al. 2005), but also promote adhesion of the CTCs to endothelium when they arrive the metastatic foci (Anvari et al. 2021).
Although no specific studies have confirmed the relationship between NETs and tumor cell-platelet aggregates, there is evidence that NETs are able to induce a procoagulant state, which may favor the formation of tumor cell-platelet aggregates. NETs (Elaskalani et al. 2018) and NET-associated molecules, such as cell-free DNA (Jansen et al. 2017), extracellular histones (Semeraro et al. 2011) and granular enzymes (Kolarova et al. 2013), were reported to trigger platelet activation and aggregation. Furthermore, NETs could provide a scaffold for the aggregation of platelets and other coagulation proteins (Fuchs et al. 2010), induce overexpression of tissue factor on endothelial cells (Folco et al. 2018; Haubitz et al. 2001; Yang et al. 2016) and inhibit fibrinolysis by degradation of plasminogen (Cruz et al. 2019). In fact, several studies have pointed out that tumor-induced NET formation plays an important role in cancer-associated thrombosis (Abdol Razak et al. 2017; Demers et al. 2012; Hisada et al. 2020; Mauracher et al. 2018).
Platelets and fibrins encoat and wrap around the CTCs, hence support their survival by impeding immune cell-mediated cytotoxicity (Palumbo et al. 2005). Likewise, CTCs have been noted to benefit from a similar protection bestowed by NETs. Teijeira et al. observed that when tumor spheroids were co-cultured with NK cells or CD8+ T cells (Teijeira et al. 2020), NETs-coated tumor spheroids grew much larger than those without NETs coating. Subsequent in vivo experiments further confirmed that NETs could impair the contact between NK cells and circulating tumor cells in liver sinusoids, and reduce CD8+ T cell infiltration into subcutaneous tumor. The inhibition of NET formation could restore the cytotoxic abilities of NK cells and reduce metastasis. Taking into account all the above findings, the coverage of NETs might protect circulating tumor cells from cytotoxic attacks during dissemination and settlement.
NETs enhance the adhesion of circulating tumor cells
If CTCs survive the perils of dissemination, they eventually attach to the walls of the microvasculature and start extravasation. The adhesion of CTCs to endothelium is not an easy process due to a rapid and turbulent blood flow. The pre-existing NETs within metastatic target organs might facilitate adhesion by capturing CTCs (Najmeh et al. 2017; Ren et al. 2021).
As mentioned earlier, inflammation could promote the formation of NETs (Albrengues et al. 2018; Cools-Lartigue et al. 2013). Mouse models subjected to cecal ligation puncture will develop extensive abdominal septic response and deposit a massive amount of NETs within hepatic sinusoids. These intravascular NETs will then bind circulating tumor cells in an integrin-dependent manner (Najmeh et al. 2017). Najmeh et al. found that β1-integrin was present both on tumor cells and NETs and they hypothesized that these common subunits might be connected through a bridging molecule, such as an ECM protein (Najmeh et al. 2017). As the expression of integrins may vary depending on the type of tumor cells, the abilities of different tumor cells to adhere to NETs were tested in vitro in another study. This study showed that the expression level of integrins α5β1, αvβ3, and αvβ5 is positively correlated with the adhesion of tumor cells to NETs (Monti et al. 2018). Moreover, NETs have been shown to be able to capture tumor-platelet aggregates in a hepatic I/R injury model (Ren et al. 2021), possibly through interactions between NETs and TLR4-activated platelets (Elaskalani et al. 2018; Semeraro et al. 2011).
A further study indicated that NETs could induce upregulation of adhesion molecules on endothelium. Cathepsins bound to NETs are able to process pro-IL-1α into active form IL-1α, which then increases the expression of VCAM-1 and ICAM-1 in human endothelial cells (Folco et al. 2018). Overexpression of ICAM-1 in liver sinusoidal endothelial cells not only facilitates the recruitment of leukocytes and monocytes, but also enhances tumor cell adhesion and angiogenesis (Benedicto et al. 2019).
NETs awaken dormant cancer cells and promote tumor growth
It is hypothesized that tumor dormancy is a strategy employed by metastatic tumor cells when they encounter a metastasis-resistant microenvironment. These quiescent cancer cells can be reactivated in response to stimuli and lead to the formation of macrometastasis. Recently, a study suggested that inflammation-induced NETs could awaken dormant breast cancer cells (Albrengues et al. 2018). In this study, NETosis was triggered by the exposure to nasal LPS instillation and tobacco smoke in a murine model. NET-bound proteases, NE and MMP9, were found to process the ECM protein laminin using NETs as a proteolysis scaffold. NET-remodeled laminins became more bioactive and bound integrin α3β1 expressed on dormant cancer cells, which drove awakening of cancer cells via FAK/ERK/MLCK/YAP signaling cascades. Moreover, the same study also showed that NETs facilitated the proliferation of metastatic breast and prostate cancer cells. In fact, there is a considerable amount of literature suggesting that NETs could directly promote the growth of tumor cells (Demers et al. 2016; Park et al. 2016).
Activation of PRR-mediated signaling is the most common strategy adopted by NETs to promote tumor growth. In diffuse large B-cell lymphoma cells (Nie et al. 2019) and colon adenocarcinoma cells (Tohme et al. 2016), NETs could exert pro-tumorigenic effects by activating TLR9 and its downstream signaling through pathways such as NF-kB and MAPK. Similar findings were noted in glioblastoma cells (Zha et al. 2020), in which NET-derived HMGB1 bound to RAGE and upregulated the NF-κB signaling. Another example is in hepatocellular carcinoma, where the activation of TLR4 and TLR9 by NETs led to upregulation of COX2, a potent inflammatory mediator, which enhanced the invasiveness of HCC cells (Yang et al. 2020b). In addition to provoking pro-inflammatory responses in tumor cells, NETs also alter the metabolism of tumor cells. PGC1α is a transcriptional coactivator that regulates the genes involved in energy metabolism. NETs could activate TLR4-mediated p38-PGC1α signaling pathway, which causes increased mitochondrial biogenesis in metastatic CRC cells and eventually contributes to tumor growth (Yazdani et al. 2019).
NETs facilitate the remodeling of cancer-associated stroma
The tumor stroma consists of non-malignant cells (e.g. fibroblasts, immune cells and endothelial cells) and non-cellular components (e.g. extracellular matrix and basement membrane). The shaping of pro-tumorigenic stroma is critical for tumor progression and metastasis (Valkenburg et al. 2018). The extracellular matrix (ECM) is secreted by stromal cells and surrounds resident cells in tissues. The major ECM components include collagen, glycoproteins and proteoglycans. The composition and organization of these ECM elements largely affect cell signaling, cell migration, tumor growth and progression (Winkler et al. 2020). Increased deposition of adhesive glycoproteins, such as tenascin and fibronectin, favor tumor cell migration and invasion. In contrast, thrombospondin-1 (TSP-1) is an ECM protein which inhibits tumor growth and angiogenesis. Xiao et al. found that NET-bound proteases could degrade TSP-1 and thus support the metastatic growth of tumor cells (Xiao et al. 2021). Aforementioned NET-mediated modification of laminin is another example suggesting that NETs could process ECM proteins to promote tumorigenesis.
Cancer-associated fibroblasts (CAFs) are one of the most abundant non-malignant cells found in the tumor stroma. CAFs not only produce ECM proteins and soluble factors, but also release enzymes (e.g. lysyl oxidase) to process ECM components (Bremnes et al. 2011). In liver and pancreas, residing stellate cells are the primary source of CAFs. Recent studies found that pancreatic ductal adenocarcinoma-induced NETs could promote the activation of pancreatic stellate cells (Miller-Ocuin et al. 2019) and hepatic stellate cells (Takesue et al. 2020). These NET-activated stellate cells are closely correlated to the growth of primary tumor and the occurrence of liver metastasis, while degradation of NETs by DNase I inhibits the recruitment of CAFs and diminishes liver metastases (Miller-Ocuin et al. 2019; Takesue et al. 2020). The effect of NETs on fibroblasts has also been verified in non-cancerous conditions. It was reported that NETs-activated lung fibroblasts and led to exacerbation of pulmonary fibrosis (Chrysanthopoulou et al. 2014; Zhang et al. 2020b) NETs also induced the differentiation of monocytes to activated fibroblasts and promoted fibrous vascular occlusions (Sharma et al. 2021). Furthermore, substances derived from fibroblasts may amplify NET formation. For example, amyloid β secreted by CAFs can drive NET formation within primary murine pancreatic, skin and lung tumors (Munir et al. 2021). Therefore, NETs and fibroblasts may form a positive-feedback loop to enhance tumor growth and disease progression.
Tumor angiogenesis can be divided into three major parts: the inflammatory phase, the proliferative phase and the remodeling phase (Whipple and Korc 2010). During the inflammatory phase, leukocytes and monocytes are recruited to the tumor, where they produce pro-angiogenic factors to guide the proliferation of endothelial cells and stromal cells. Tumor-associated vessels are formed either by sprouting from pre-existing vessels locally or by recruiting endothelial progenitor cells from bone marrow (Chouaib et al. 2010). The early stage of tumor angiogenesis is characterized by destabilization and hyperpermeability (Whipple and Korc 2010). As discussed above, NETs may intensify and accelerate this process by inducing endothelial cell dysfunction and local basement membrane degradation. Furthermore, NETs could directly exert a pro-angiogenic activity on endothelial cells (Aldabbous et al. 2016; Jung et al. 2019; Yuan et al. 2020). Aldabbous et al. found that the stimulation of NETs could significantly promote the proliferation of human pulmonary artery endothelial cells, as shown by enhanced in vitro tube formation and spheroid sprouting, as well as increased plug vascularization in vivo (Aldabbous et al. 2016). The pro-angiogenic effect of NETs is also reflected in tumor angiogenesis. Mice receiving co-implantation of HCC cells and NETs showed a stronger expression of CD31, a reliable and widely adopted angiogenic marker, compared with mice implanted with HCC cells alone (Yang et al. 2020b). In another similar study, liver ischemia–reperfusion injury was introduced to induce NET formation before the injection of MC38 tumor cells. The tumor growth and expression of CD31 were largely reduced when the mice were also treated with NETs inhibitor DNase, compared to non-treated group (Tohme et al. 2016).
The interaction between NETs and immune cells
Elevated neutrophil-to-lymphocyte ratio and increasing neutrophil infiltration have been linked to poor prognosis in various malignancies, including colorectal cancer (Halazun et al. 2008), hepatocellular carcinoma (Margetts et al. 2018) and breast cancer (Corbeau et al. 2020). Apart from releasing NETs, infiltrating neutrophils are able to exert multiple pro-tumorigenic effects (Wang et al. 2018). Interestingly, recent study showed that NETs could interact with neutrophils to form a positive-feedback loop. NETs upregulate β2 integrin expression on neutrophils (which bind onto endothelial ICAM-1), and promote the translocation of P-selectins on endothelial cells (which interact with neutrophil PSGL-1) (Lavoie et al. 2018). These two concurrent changes significantly facilitate the rolling and adhesion of leukocytes. Furthermore, NETs may modulate the recruitment of immune cells by adjusting the concentration of local inflammatory mediators. Researchers have found that NET-associated proteases may selectively degrade cytokines and chemokines (Hahn et al. 2019; Schauer et al. 2014), including IL-1β, IL-6, monocyte chemoattractant protein-1, macrophage inflammatory proteins and TNF. In contrast, the IL-8, a potent neutrophil attractant and NETs stimulator, is degraded to a lesser extent (Schauer et al. 2014). In fact, several studies have pointed out that NETs could upregulate IL-8 expression and secretion (Dömer et al. 2021; Hudock et al. 2020; Zha et al. 2020), which might attract more neutrophils to deposit additional NETs in disease foci. In addition to playing a role in the recruitment of neutrophils, NETs may further boost the pro-inflammatory activities of neutrophils, such as promoting their ROS production and phagocytosis (Dömer et al. 2021).
CD8+ T cells are important effector lymphocytes responsible for the anti-cancer immune response. Checkpoint molecules, such as CTLA4 and PD-1, are immune inhibitory proteins which act as an "off switch" to prevent T cells from attacking other cells. As such, checkpoint blockade immunotherapy could enhance the cytotoxic effect of T cells on tumor cells. Recent studies found that NETs are associated with resistance to checkpoint inhibitor treatment in pancreatic cancer (Zhang et al. 2020c) and colorectal cancer (Zhang et al. 2021). Inhibition of NETs by systemic administration of DNase I or the use of a PAD4−/− model, largely improves the efficacy of anti-PD-1 immunotherapy, which is manifested by reduced tumor growth, increased tumor-associated CD8+ T cell infiltration and cytotoxicity. A previous study has pointed out that NETs may act as a barrier to access and prevent cytotoxic immune cells from approaching tumor cells effectively (Teijeira et al. 2020), which may explain why NETs cause resistance to anti-PD-1 therapy.
Although little knowledge exists about the crosstalk between NETs and tumor-associated immune cells, the impact of NETs on immune cells has been well documented in the context of autoimmune disease. A series of studies have revealed that NETs contribute to the pathogenesis of autoimmune disease by interacting with local immune cells, including macrophages (Krishnamoorthy et al. 2018; Warnatsch et al. 2015), plasmacytoid dendritic cells (Garcia-Romo et al. 2011; Parackova et al. 2020; Qiu et al. 2017; Villanueva et al. 2011) and T lymphocytes (Lambert et al. 2019; Tillack et al. 2012). For example, NETs can accelerate the disease progression of SLE by through the stimulation of macrophages and the resultant upregulation of the STING pathway, which is also extensively involved in tumor growth and metastasis (Ahn et al. 2014; Lemos et al. 2016; Liang et al. 2015; Song et al. 2017). It is very likely that these findings, regarding NET-triggered signaling pathways discovered in inflammation, could also be applicable in the scenario of carcinogenesis.
The potential therapeutic applications of NETs
Since NETs play an influential role in tumor growth and metastasis, targeting NETs could be a promising and novel approach against cancer. A number of pre-clinical models have demonstrated that inhibiting NETosis or inhibiting NET-mediated action could improve disease progression. In the following section, we will discuss how these NET inhibitors (Table 3) can be applied into clinical practice.
The inhibitors of NET formation
Targeting first messengers and their receptors is a widely used therapeutic strategy, which can also be applied to reduce NET formation and NET-mediated changes. IL-8 secreted by tumor cells induces lytic NETosis via the CXCR2-mediated pathway. Treatment with reparixin, an inhibitor of CXCR1 and CXCR2, largely decreases the intra-tumoral NET formation in breast cancer-bearing mice (Teijeira et al. 2020). Another study also showed that CXCR2 inhibition significantly reduced NET-primed tumor growth in lymphoma-bearing mice (Nie et al. 2019). More proof was obtained in studies on infectious diseases and chronic inflammatory conditions, in which C–X–C chemokine receptor antagonists successfully inhibits NETosis and alleviates inflammation (Pedersen et al. 2018). Another mechanism of lytic NETosis was mediated by immune complex and Fcγ receptor. Fostamatinib, a FDA-approved oral agent for the treatment of chronic idiopathic thrombocytopenic purpura, is able to disrupt FcγR-mediated signaling and inhibit NET formation in vitro (Strich et al. 2021a, b). Moreover, a phase II study targeting COVID-19 showed that plasma NETs decreased more rapidly in patients receiving fostamatinib compared with those receiving placebo (Strich, Tian, et al. 2021). Besides tumor-derived cytokines and immune complexes, several PAMPs and DAMPs, such as LPS and HMGB1, are considered potent inducers of NOX-independent NETosis. Taurolidine, an antimicrobial drug which denatures LPS, is able to decrease tobacco smoke-induced NET formation and attenuate NET-related activation of quiescent cancer cells (Albrengues et al. 2018). Similarly, the administration of anti-HMGB1 antibodies effectively decreases NET formation in vitro and in vivo (Kim et al. 2019; Tadie et al. 2013).
ROS is an essential second messenger involved in classical NET formation. Neutrophils isolated from patients with chronic granulomatous disease (CGD), a disease that renders patients unable to produce enough ROS due to NADPH oxidase deficiency, fail to release NETs if they are stimulated with PMA or S. aureus (Fuchs et al. 2007). Likewise, the inhibition of NADPH oxidase by diphenyleneiodonium (Fuchs et al. 2007) or apocynin (Takesue et al. 2020), dramatically decreases NET formation in vitro. Although the clinical validity of these NADPH inhibitors is largely unknown, the biosafety and pharmacokinetics of setanaxib (oral administration) and apocynin (nebulization) have been confirmed in phase I and phase II studies (Elbatreek et al. 2020; Stefanska et al. 2012). Metformin, a first-line medication for diabetes, inhibits the PKC-NADPH oxidase axis by reducing the membrane translocation of PKC-βII (Batchuluun et al. 2014). Metformin was shown to completely block PMA-induced NETosis in vitro. In patients who received metformin therapy, the concentration of plasma NET biomarkers, including cell-free DNA, NE, PR3, were significantly reduced (Menegazzo et al. 2018).
NE and PAD4 are two key enzymes which regulate NET formation. Both enzymes contribute to histone modification and chromatin decondensation. In addition to histone cleavage, NET-bound NE may interact with target cells to provoke a pro-inflammatory or procoagulant state. As such, the administration of NE inhibitors sivelestat, not only prevents NETosis, but also limits NET-mediated cytotoxicity and response (Okeke et al. 2020; Zhou et al. 2020). Sivelestat has been clinically tested in thoracoscopic esophagectomy for esophageal cancer and successfully reduced the incidence of post-operative acute lung injury and decreased surgery-induced pulmonary function loss (Makino et al. 2011). As mentioned before, PAD4 is another essential enzyme involved in NETosis. PAD4 knockout mice are widely used as models to study the impact of the absence of NETs. Without the presence of NETs, tumors often grow slower in PAD4 knockout mice compared to wild type controls (Yazdani et al. 2019). Similarly, the pharmacological inhibition of PAD4 with chloramidine (Li et al. 2010; Park et al. 2016) or GSK484 (Teijeira et al. 2020) also reduces NET production and ameliorates the disease progression in various models of autoimmune and infectious disease.
Although pre-clinical models have proven the effectiveness of the NET formation inhibitors mentioned above, it is worth noting that many of the targets, such as NE and NADPH oxidase, are integral components of neutrophils’ antibacterial functions. It remains to be studied whether inhibiting these enzymes increases the risk of infection.
Blocking NET-mediated action
DNAse could effectively remove NETs by degrading their DNA backbones. Several in vivo studies have demonstrated that DNase treatment could prominently reduce NET-mediated tumor growth and metastasis (Cools-Lartigue et al. 2013; Miller-Ocuin et al. 2019; Nie et al. 2019; Park et al. 2016; Takesue et al. 2020; Teijeira et al. 2020; Tohme et al. 2016; Yang et al. 2020a, b). For example, intravenous administration of DNAse could minimize sepsis-induced NETs and attenuate hepatic metastases in vivo (Cools-Lartigue et al. 2013; Tohme et al. 2016). The biosafety of DNase has already been tested in clinical trials for indications other than cancer. Dornase alfa is a commercially available recombinant human DNase. Nebulization of dornase alfa ameliorates the disease progression of cystic fibrosis by reducing the viscosity of the sputum and reduces post-operative lower respiratory tract infection in patients receiving lung transplants (Tarrant et al. 2019). NETs are frequently found in various pathological conditions of the lower respiratory tract (Hudock et al. 2020; Pandolfi et al. 2021; Tadie et al. 2013; Zhang et al. 2020b, the inhalation of DNase could be a solution to reduce NET-related tissue damage and minimize local inflammation. Moreover, some clinical trials tested the use of DNase as adjuvant therapy against leukemia (NCT02462265) or head and neck cancer (NCT00536952). Oklu et al. suggested that the reduced levels and activities of endogenous nuclease were responsible for increased NETs in the circulation of cancer patients (Oklu et al. 2017). Therefore, DNase supplementation could be an efficient therapeutic strategy to reduce tumor-associated NET formation and NET-mediated pro-metastatic responses.
TLRs are the most studied receptors involved in NET recognition and signal initiation. The effects of NETs may diminish when target cells are transfected with TLR siRNA or treated with specific antagonists. For examples, silencing of TLR2 attenuates NET-mediated endothelial cell activation (Quillard et al. 2015), blockage of TLR4 by siRNA and Eritoran reduces mitochondrial biogenesis in MC38 colon adenocarcinoma cells (Yazdani et al. 2019), blockage of endosomal TLR9 by oligonucleotide decreases the NET-induced proliferation in SU-DHL-2 lymphoma cells (Nie et al. 2019). NET-mediated pro-inflammatory response largely vanishes in human hepatocellular cells when TLR4 and TLR9 are both knocked out (Yang et al. 2020b). Although a series of in vitro experiments have proved that TLRs could suppress NET-mediated actions, more studies need to be done to evaluate the effectiveness of TLR inhibition in vivo.
Von Willebrand factor (VWF) is an adhesive glycoprotein expressed on endothelial cells and Kupffer cells (Wong et al. 2013). Some studies have demonstrated that VWF serves as a binding site for the DNA component of NETs, which allows the attachment of NETs to the lumen of vessels (Grässle et al. 2014; Kolaczkowska et al. 2015). Intravascular administration of ADAMST13, a VWF protease, reduces VWF-dependent NET adherence to the vascular wall and minimizes their damage to liver (Kolaczkowska et al. 2015). Recombinant ADAMTS-13 has been shown to be safe and well-tolerated in clinical trials for hereditary thrombotic thrombocytopenic purpura (Scully et al. 2017), so it could perhaps be used to remove intravascular NETs and diminish NET-mediated tumor cell adhesion in the future. Furthermore, unfractionated heparin could strongly accelerate NET degradation in vitro by suppressing VWF-NETs interaction (Grässle et al. 2014; Leppkes et al. 2020), whilst low-molecule-weight heparin was less effective in clearing NETs (Grässle et al. 2014). The appropriate administration of unfractioned heparin may reduce the NETosis caused by surgical interventions and benefit the patients.
Aspirin is one of the most commonly used anti-inflammatory and anti-platelet agents. The anti-platelet effect of aspirin may protect patients against metastasis and relapse. In our previous study, we found that perioperative aspirin intake significantly prolonged the disease-free period in patients who received curative resection for pancreatic cancer (Pretzsch et al. 2021). Interestingly, in vitro and in vivo models also show that aspirin could impair NET formation by impeding NF-κB activation (Lapponi et al. 2013). As we discussed above, the interaction between NETs and platelets is bi-directional. Activated platelets could be a potent inducer of NETosis, and NETs in turn could further enhance the activation and pro-metastatic actions of platelets. Thereby, the use of aspirin may prevent both NET formation and NET-platelet interactions. Unlike other NASIDs, aspirin has a weak inhibitory role on COX-2, a key enzyme involved in NETs-mediated inflammatory response in HCC (Yang et al. 2020b) and breast cancer (Martins-Cardoso et al. 2020). Celecoxib, a selective COX-2 inhibitor, could reduce the NET-enhanced invasion capacity of HCC cells in vitro (Yang et al. 2020b). More research is still needed to determine how NSAIDs interact with NETs, and whether or not they can be effectively used against NETs in routine practice.
Certain medical interventions may increase the risk of developing NETosis and cause NET-related adverse effects. Granulocyte-colony stimulating factor (G-CSF) injection is a standard treatment for hematopoietic stem-cell transplantation and chemotherapy-induced neutropenia. G-CSF not only stimulates the proliferation of neutrophils, but also induces them to release NETs (Demers et al. 2016; Giaglis et al. 2016; Schoergenhofer et al. 2017). NET formation induced by G-CSF treatment significantly promotes tumor growth in vivo (Demers et al. 2016). Another example is radiotherapy in bladder cancer. Shinde-Jadhav et al. found that HMGB1 released during radiation eventually led to NET formation, which may contribute to radiation resistance (Shinde-Jadhav et al. 2021). Besides medical treatment, surgical operations also predispose NET formation (Beaubien-Souligny et al. 2019; Cools-Lartigue et al. 2013; Ren et al. 2021; Tohme et al. 2016; von Meijenfeldt et al. 2018). Curative surgery is the frontline therapy for patients with resectable tumors, however, relapses and distant metastases are common even after an uneventful operation. The NETs formed during the surgery may create a pre-metastatic niche which favors the implantation of circulating tumor cells (Cools-Lartigue et al. 2013; Ren et al. 2021; Tohme et al. 2016). Several in vivo studies showed intravenous administration of DNase could diminish surgical-related NET deposition and attenuate the distant metastases (Cools-Lartigue et al. 2013; Tohme et al. 2016). Prophylactic use of NET inhibitors, such as DNase, may reduce undesired effects caused by NETs and improve therapeutic outcomes.
Conclusion
Neutrophils release NETs as an innate immune response to various infectious and inflammatory stimuli. Although one of the main purposes of NET formation is to counter microbial invasion, it also plays pathological roles in tumorigenesis and metastasis. NETs consist of various damage-associated molecular patterns, which can be recognized by a series of pattern recognition receptors and initiate downstream signaling. There is plenty of evidence that NETs interact directly with cancer cells. These interactions may promote tumor cell proliferation, induce epithelial–mesenchymal transition, facilitate adhesion of circulating tumor cells or awaken dormant cancer cells. The evidence also showed that NETs might modulate the tumor microenvironment by degrading anti-tumorigenic ECM elements, activating cancer-associated fibroblasts and inhibiting the cytotoxicity of tumor-associated immune cells. The deposition of NETs in distant organs may create a pre-metastatic niche to host the circulating tumor cells, and thus promote distant metastasis. Circulating NET markers, including H3Cit and MPO-dsDNA, although not specific for cancer-associated NET formation, might be useful to detect relevant NETosis and thus high risk of metastasis. Although the pro-tumorigenic roles of NETs have been widely recognized, therapeutic strategies targeting NETs still need to be developed. Several NET inhibitors, including DNase, have been tested in vivo or in early clinical trials for other indications. Considering the role of NETs in tumor progression, targeting NETs could be a promising diagnostic and therapeutic approach for cancer management.
References
Abdol Razak N, Elaskalani O, Metharom P (2017) Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. Int J Mol Sci 18(3):487
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158(5):1110–1122
Ahn J, Xia T, Konno H, Konno K, Ruiz P, Barber GN (2014) Inflammation-driven carcinogenesis is mediated through STING. Nat Commun 5(1):1–9
Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V (2018) Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science, 361(6409).
Aldabbous L, Abdul-Salam V, McKinnon T, Duluc L, Pepke-Zaba J, Southwood M, Ainscough AJ, Hadinnapola C, Wilkins MR, Toshner M (2016) Neutrophil extracellular traps promote angiogenesis: evidence from vascular pathology in pulmonary hypertension. Arterioscler Thromb Vasc Biol 36(10):2078–2087
Alemán OR, Mora N, Cortes-Vieyra R, Uribe-Querol E, Rosales C (2016b) Transforming growth factor-β-activated kinase 1 is required for human FcγRIIIb-induced neutrophil extracellular trap formation. Front Immunol 7:277
Alemán OR, Mora N, Cortes-Vieyra R, Uribe-Querol E, Rosales C (2016a) Differential use of human neutrophil Fcγ receptors for inducing neutrophil extracellular trap formation. J Immunol Res 2016a.
An Z, Li J, Yu J, Wang X, Gao H, Zhang W, Wei Z, Zhang J, Zhang Y, Zhao J (2019) Neutrophil extracellular traps induced by IL-8 aggravate atherosclerosis via activation NF-κB signaling in macrophages. Cell Cycle 18(21):2928–2938
Anvari S, Osei E, Maftoon N (2021) Interactions of platelets with circulating tumor cells contribute to cancer metastasis. Sci Rep 11(1):1–16
Apel F, Andreeva L, Knackstedt LS, Streeck R, Frese CK, Goosmann C, Hopfner K-P, Zychlinsky A (2021) The cytosolic DNA sensor cGAS recognizes neutrophil extracellular traps. Sci Signaling, 14(673).
Ashar HK, Pulavendran S, Rudd JM, Maram P, Achanta M, Chow VTK, Malayer JR, Snider TA, Teluguakula N (2021) Administration of a CXC chemokine receptor 2 (CXCR2) antagonist, SCH527123, together with oseltamivir suppresses netosis and protects mice from lethal influenza and piglets from swine-influenza infection. Am J Pathol 191(4):669–685. https://doi.org/10.1016/j.ajpath.2020.12.013
Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, Miura D, Takayanagi R (2014) Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD (P) H oxidase pathway in human aortic endothelial cells. Atherosclerosis 232(1):156–164
Beaubien-Souligny W, Neagoe P-E, Gagnon D, Denault AY, Sirois MG (2019) Increased circulating levels of neutrophil extracellular traps during cardiopulmonary bypass. CJC Open 2(2):39–48. https://doi.org/10.1016/j.cjco.2019.12.001
Benedicto A, Herrero A, Romayor I, Marquez J, Smedsrød B, Olaso E, Arteta B (2019) Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Sci Rep 9(1):1–12
Binet F, Cagnone G, Crespo-Garcia S, Hata M, Neault M, Dejda A, Wilson AM, Buscarlet M, Mawambo GT, Howard JP (2020) Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science, 369(6506).
Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, Singhi AD, Kang R, Tang D, Lotze MT (2015) The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther 22(6):326–334
Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 15(11):1017–1025
Bremnes RM, Dønnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund L-T (2011) The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol 6(1):209–217
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil Extracellular Traps Kill Bacteria Science 303(5663):1532–1535
Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ (2015) Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 74(7):1417–1424
Chen K, Murao A, Arif A, Takizawa S, Jin H, Jiang J, Aziz M, Wang P (2021) Inhibition of efferocytosis by extracellular CIRP–induced neutrophil extracellular traps. J Immunol 206(4):797–806
Chouaib, S., Kieda, C., Benlalam, H., Noman, M. Z., Mami-Chouaib, F., & Ruegg, C. (2010). Endothelial cells as key determinants of the tumor microenvironment: interaction with tumor cells, extracellular matrix and immune killer cells. Critical Reviews™ in Immunology, 30(6).
Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, Sivridis E, Koffa M, Giatromanolaki A, Boumpas DT (2014) Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol 233(3):294–307
Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD (2007) Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13(4):463–469
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig 123(8):3446–3458
Corbeau I, Jacot W, Guiu S (2020) Neutrophil to lymphocyte ratio as prognostic and predictive factor in breast cancer patients: a systematic review. Cancers 12(4):958
Cruz DBd, Helms J, Aquino LR, Stiel L, Cougourdan L, Broussard C, Chafey P, Riès‐Kautt M, Meziani F, Toti F (2019) DNA‐bound elastase of neutrophil extracellular traps degrades plasminogen, reduces plasmin formation, and decreases fibrinolysis: proof of concept in septic shock plasma. The FASEB Journal, 33(12), 14270-14280.
Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD (2012) Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci 109(32):13076–13081
Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, Wagner DD (2016) Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 5(5):e1134073
Dicker AJ, Crichton ML, Pumphrey EG, Cassidy AJ, Suarez-Cuartin G, Sibila O, Furrie E, Fong CJ, Ibrahim W, Brady G (2018) Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 141(1):117–127
Dömer D, Walther T, Möller S, Behnen M, Laskay T (2021) Neutrophil extracellular traps activate proinflammatory functions of human neutrophils. Front Immunol 12:1190
Dongre A, Weinberg RA (2019) New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 20(2):69–84
Douda DN, Khan MA, Grasemann H, Palaniyar N (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci 112(9):2817–2822
Dyer MR, Chen Q, Haldeman S, Yazdani H, Hoffman R, Loughran P, Tsung A, Zuckerbraun BS, Simmons RL, Neal MD (2018) Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep 8(1):2068. https://doi.org/10.1038/s41598-018-20479-x
Egan K, Cooke N, Kenny D (2014) Living in shear: platelets protect cancer cells from shear induced damage. Clin Exp Metas 31(6):697–704
Elaskalani O, Razak NBA, Metharom P (2018) Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones. Cell Commun Signaling 16(1):1–15
Elbatreek MH, Mucke H, Schmidt HH (2020) NOX inhibitors: from bench to naxibs to bedside.
Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P (2018) Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler Thromb Vasc Biol 38(8):1901–1912
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176(2):231–241
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci 107(36):15880–15885
Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL (2011) Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Trans Med 3(73), 73ra20–73ra20.
Giaglis S, Stoikou M, Sur Chowdhury C, Schaefer G, Grimolizzi F, Rossi SW, Hoesli IM, Lapaire O, Hasler P, Hahn S (2016) Multimodal regulation of NET formation in pregnancy: progesterone antagonizes the pro-NETotic effect of estrogen and G-CSF. Front Immunol 7:565
Grässle S, Huck V, Pappelbaum KI, Gorzelanny C, Aponte-Santamaría C, Baldauf C, Gräter F, Schneppenheim R, Obser T, Schneider SW (2014) von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler Thromb Vasc Biol 34(7):1382–1389
Gray RD, Lucas CD, MacKellar A, Li F, Hiersemenzel K, Haslett C, Davidson DJ, Rossi AG (2013) Activation of conventional protein kinase C (PKC) is critical in the generation of human neutrophil extracellular traps. J Inflamm 10(1):1–8
Guan X, Lu Y, Zhu H, Yu S, Zhao W, Chi X, Xie C, Yin Z (2021) The crosstalk between cancer cells and neutrophils enhances hepatocellular carcinoma metastasis via neutrophil extracellular traps-associated cathepsin g component: a potential therapeutic target. Journal of Hepatocellular Carcinoma 8:451
Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ (2010) Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 584(14):3193–3197
Hahn J, Schauer C, Czegley C, Kling L, Petru L, Schmid B, Weidner D, Reinwald C, Biermann MH, Blunder S (2019) Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J 33(1):1401–1414
Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H (2011) Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol 7(2):75–77
Halazun K, Aldoori A, Malik H, Al-Mukhtar A, Prasad K, Toogood G, Lodge J (2008) Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Euro J Surg Oncol (EJSO) 34(1):55–60
Haubitz M, Gerlach M, Kruse H, Brunkhorst R (2001) Endothelial tissue factor stimulation by proteinase 3 and elastase. Clin Exp Immunol 126(3):584–588
Heeke S, Mograbi B, Alix-Panabières C, Hofman P (2019) Never travel alone: the crosstalk of circulating tumor cells and the blood microenvironment. Cells 8(7):714
Hisada Y, Grover SP, Maqsood A, Houston R, Ay C, Noubouossie DF, Cooley BC, Wallén H, Key NS, Thålin C (2020) Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica 105(1):218
Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, Tsung A (2015) Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 62(2):600–614. https://doi.org/10.1002/hep.27841
Hudock KM, Collins MS, Imbrogno M, Snowball J, Kramer EL, Brewington JJ, Gollomp K, McCarthy C, Ostmann AJ, Kopras EJ (2020) Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia. Am J Physiol-Lung Cell Mol Physiol 319(1):L137–L147
Jansen MP, Emal D, Teske GJ, Dessing MC, Florquin S, Roelofs JJ (2017) Release of extracellular DNA influences renal ischemia reperfusion injury by platelet activation and formation of neutrophil extracellular traps. Kidney Int 91(2):352–364
Jin W, Yin H, Li H, Yu XJ, Xu HX, Liu L (2021) Neutrophil extracellular DNA traps promote pancreatic cancer cells migration and invasion by activating EGFR/ERK pathway. Journal of cellular and molecular medicine.
Jung HS, Gu J, Kim J-E, Nam Y, Song JW, Kim HK (2019) Cancer cell–induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS ONE 14(4):e0216055
Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ (2013) Neutrophil extracellular trap–associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 190(3):1217–1226
Kajioka H, Kagawa S, Ito A, Yoshimoto M, Sakamoto S, Kikuchi S, Kuroda S, Yoshida R, Umeda Y, Noma K (2021) Targeting neutrophil extracellular traps with thrombomodulin prevents pancreatic cancer metastasis. Cancer Lett 497:1–13
Kang L, Yu H, Yang X, Zhu Y, Bai X, Wang R, Cao Y, Xu H, Luo H, Lu L (2020) Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat Commun 11(1):1–15
Kaplan MJ, Radic M (2012) Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 189(6):2689–2695
Katsuno Y, Lamouille S, Derynck R (2013) TGF-β signaling and epithelial–mesenchymal transition in cancer progression. Curr Opin Oncol 25(1):76–84
Kim S-W, Lee H, Lee H-K, Kim I-D, Lee J-K (2019) Neutrophil extracellular trap induced by HMGB1 exacerbates damages in the ischemic brain. Acta Neuropathol Commun 7(1):1–14
Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee W-Y, Sanz M-J, Mowen K, Opdenakker G, Kubes P (2015) Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun 6(1):1–13
Kolarova H, Klinke A, Kremserová S, Adam M, Pekarová M, Baldus S, Eiserich JP, Kubala L (2013) Myeloperoxidase induces the priming of platelets. Free Radical Biol Med 61:357–369
Krishnamoorthy N, Douda DN, Brüggemann TR, Ricklefs I, Duvall MG, Abdulnour R-E E, Martinod K, Tavares L, Wang X, Cernadas M (2018). Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol 3(26), eaao4747.
Lambert S, Hambro CA, Johnston A, Stuart PE, Tsoi LC, Nair RP, Elder JT (2019) Neutrophil extracellular traps induce human Th17 cells: effect of psoriasis-associated TRAF3IP2 genotype. J Investig Dermatol 139(6):1245–1253
Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang Y-H, Homey B, Cao W, Wang Y-H, Su B, Nestle FO (2007) Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449(7162):564–569
Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V (2011) Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Science Trans Med, 3(73), 73ra19–73ra19.
Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, Pozner RG, Schattner M (2013) Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther 345(3):430–437
Lavoie SS, Dumas E, Vulesevic B, Neagoe PE, White M, Sirois MG (2018) Synthesis of human neutrophil extracellular traps contributes to angiopoietin-mediated in vitro proinflammatory and proangiogenic activities. J Immunol 200(11):3801–3813. https://doi.org/10.4049/jimmunol.1701203
Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, Munn D, Mellor AL (2016) STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Can Res 76(8):2076–2081
Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Stürzl M, Staats L, Mahajan A, Schauer C (2020) Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 58:102925
Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y (2010) PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular trapsPAD4 in NET-mediated bacterial killing. J Exp Med 207(9):1853–1862
Li Y, Li M, Weigel B, Mall M, Werth VP, Liu ML (2020) Nuclear envelope rupture and NET formation is driven by PKCα-mediated lamin B disassembly. EMBO Rep 21(8):e48779
Liang D, Xiao-Feng H, Guan-Jun D, Er-Ling H, Sheng C, Ting-Ting W, Qin-Gang H, Yan-Hong N, & Ya-Yi H (2015) Activated STING enhances Tregs infiltration in the HPV-related carcinogenesis of tongue squamous cells via the c-jun/CCL22 signal. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1852(11), 2494–2503.
Lim CH, Adav SS, Sze SK, Choong YK, Saravanan R, Schmidtchen A (2018) Thrombin and plasmin alter the proteome of neutrophil extracellular traps. Front Immunol 9:1554
Liu L, Mao Y, Xu B, Zhang X, Fang C, Ma Y, Men K, Qi X, Yi T, Wei Y, Wei X (2019) Induction of neutrophil extracellular traps during tissue injury: Involvement of STING and Toll-like receptor 9 pathways. Cell Prolif 52(3):e12579–e12579. https://doi.org/10.1111/cpr.12579
Makino H, Kunisaki C, Kosaka T, Akiyama H, Morita S, Endo I (2011) Perioperative use of a neutrophil elastase inhibitor in video-assisted thoracoscopic oesophagectomy for cancer. J British Surgery 98(7):975–982
Margetts J, Ogle LF, Chan SL, Chan AW, Chan KA, Jamieson D, Willoughby CE, Mann DA, Wilson CL, Manas DM (2018) Neutrophils: driving progression and poor prognosis in hepatocellular carcinoma? Br J Cancer 118(2):248–257
Martins-Cardoso K, Almeida VH, Bagri KM, Rossi MID, Mermelstein CS, König S, Monteiro RQ (2020) Neutrophil extracellular traps (Nets) promote pro-metastatic phenotype in human breast cancer cells through epithelial–mesenchymal transition. Cancers 12(6):1542
Mauracher LM, Posch F, Martinod K, Grilz E, Däullary T, Hell L, Brostjan C, Zielinski C, Ay C, Wagner D (2018) Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thromb Haemost 16(3):508–518
Menegazzo L, Scattolini V, Cappellari R, Bonora BM, Albiero M, Bortolozzi M, Romanato F, Ceolotto G, de Kreutzeberg SV, Avogaro A (2018) The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol 55(6):593–601
Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V (2014) A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep 8(3):883–896
Miller-Ocuin JL, Liang X, Boone BA, Doerfler WR, Singhi AD, Tang D, Kang R, Lotze MT, Zeh HJ III (2019) DNA released from neutrophil extracellular traps (NETs) activates pancreatic stellate cells and enhances pancreatic tumor growth. Oncoimmunology 8(9):e1605822
Monti M, De Rosa V, Iommelli F, Carriero MV, Terlizzi C, Camerlingo R, Belli S, Fonti R, Di Minno G, Del Vecchio S (2018) Neutrophil extracellular traps as an adhesion substrate for different tumor cells expressing RGD-binding integrins. Int J Mol Sci 19(8):2350
Munir H, Jones JO, Janowitz T, Hoffmann M, Euler M, Martins CP, Welsh SJ, Shields JD (2021) Stromal-driven and Amyloid β-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat Commun 12(1):1–16
Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau S, Ferri LE (2017) Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer 140(10):2321–2330
Neubert E, Meyer D, Rocca F, Günay G, Kwaczala-Tessmann A, Grandke J, Senger-Sander S, Geisler C, Egner A, Schön MP (2018) Chromatin swelling drives neutrophil extracellular trap release. Nat Commun 9(1):1–13
Neumann A, Völlger L, Berends ET, Molhoek EM, Stapels DA, Midon M, Friães A, Pingoud A, Rooijakkers SH, Gallo RL (2014) Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun 6(6):860–868
Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, Liu P, Li Z, Xia Y, Jiang W (2019) Neutrophil extracellular traps induced by IL8 promote diffuse large B-cell lymphoma progression via the TLR9 signaling. Clin Cancer Res 25(6):1867–1879
Okeke EB, Louttit C, Fry C, Najafabadi AH, Han K, Nemzek J, Moon JJ (2020) Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 238:119836
Oklu R, Sheth RA, Wong KH, Jahromi AH, Albadawi H (2017) Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovasc Diagnosis Therapy 7(Suppl 3):S140
Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL (2005) Platelets and fibrin (ogen) increase metastatic potential by impeding natural killer cell–mediated elimination of tumor cells. Blood 105(1):178–185
Pandolfi L, Bozzini S, Frangipane V, Percivalle E, De Luigi A, Violatto MB, Lopez G, Gabanti E, Carsana L, D’Amato M (2021) Neutrophil extracellular traps induce the epithelial-mesenchymal transition: implications in post-COVID-19 fibrosis. Front Immunol 12:2321
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191(3):677–691
Parackova Z, Zentsova I, Vrabcova P, Klocperk A, Sumnik Z, Pruhova S, Petruzelkova L, Hasler R, Sediva A (2020) Neutrophil extracellular trap induced dendritic cell activation leads to Th1 polarization in type 1 diabetes. Front Immunol 11:661
Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH (2016) Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Science translational medicine, 8(361), 361ra138–361ra138.
Pedersen F, Waschki B, Marwitz S, Goldmann T, Kirsten A, Malmgren A, Rabe KF, Uddin M, Watz H (2018) Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Euro Respiratory J 51(4).
Pieterse E, Rother N, Yanginlar C, Hilbrands LB, van der Vlag J (2016) Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Front Immunol 7:484
Pieterse E, Rother N, Garsen M, Hofstra JM, Satchell SC, Hoffmann M, Loeven MA, Knaapen HK, van der Heijden OW, Berden JH (2017) Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol 37(7):1371–1379
Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185(12):7413–7425
Pretzsch E, D’Haese J, Renz B, Ilmer M, Schiergens T, Miksch R, Albertsmeier M, Guba M, Angele M, Werner J (2021) Effect of platelet inhibition with perioperative aspirin on survival in patients undergoing curative resection for pancreatic cancer: a propensity score matched analysis. BMC Surg 21(1):1–10
Qiu S-L, Zhang H, Tang Q-Y, Bai J, He Z-Y, Zhang J-Q, Li M-H, Deng J-M, Liu G-N, Zhong X-N (2017) Neutrophil extracellular traps induced by cigarette smoke activate plasmacytoid dendritic cells. Thorax 72(12):1084–1093
Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P (2015) TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J 36(22):1394–1404
Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N (2019). Primary tumors induce neutrophil extracellular traps with targetable metastasis-promoting effects. JCI insight, 4(16).
Regmi S, Fu A, Luo KQ (2017) High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep 7(1):1–12
Ren J, He J, Zhang H, Xia Y, Hu Z, Loughran P, Billiar T, Huang H, Tsung A (2021) Platelet TLR4-ERK5 axis facilitates NET-mediated capturing of circulating tumor cells and distant metastasis after surgical stress. Can Res 81(9):2373–2385
Rohrbach A, Slade D, Thompson P, Mowen K (2012) Activation of PAD4 in NET formation. Front Immunol 3:360
Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT (2012) Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7(2):e32366
Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E (2014) Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20(5):511–517
Schoergenhofer C, Schwameis M, Wohlfarth P, Brostjan C, Abrams ST, Toh C-H, Jilma B (2017) Granulocyte colony-stimulating factor (G-CSF) increases histone-complexed DNA plasma levels in healthy volunteers. Clin Exp Med 17(2):243–249
Scully M, Knöbl P, Kentouche K, Rice L, Windyga J, Schneppenheim R, Hovinga JAK, Kajiwara M, Fujimura Y, Maggiore C (2017) Recombinant ADAMTS-13: first-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood J Am Soc Hematol 130(19):2055–2063
Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT (2011) Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood J Am Soc Hematol 118(7):1952–1961
Sharma S, Hofbauer TM, Ondracek AS, Chausheva S, Alimohammadi A, Artner T, Panzenboeck A, Rinderer J, Shafran I, Mangold A (2021) Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood J Am Soc Hematol 137(8):1104–1116
Shinde-Jadhav S, Mansure JJ, Rayes RF, Marcq G, Ayoub M, Skowronski R, Kool R, Bourdeau F, Brimo F, Spicer J, Kassouf W (2021) Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun 12(1):2776. https://doi.org/10.1038/s41467-021-23086-z
Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B (2018) Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3(26).
Song S, Peng P, Tang Z, Zhao J, Wu W, Li H, Shao M, Li L, Yang C, Duan F (2017) Decreased expression of STING predicts poor prognosis in patients with gastric cancer. Sci Rep 7(1):1–13
Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, Lotze MT (2009) RAGE (receptor for advanced glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7(1):17. https://doi.org/10.1186/1479-5876-7-17
Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, Pawliczak R (2012) Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp Lung Res 38(2):90–99
Strich JR, Ramos-Benitez MJ, Randazzo D, Stein SR, Babyak A, Davey RT, Suffredini AF, Childs RW, Chertow DS (2021a) Fostamatinib inhibits neutrophils extracellular traps induced by COVID-19 patient plasma: a potential therapeutic. J Infect Dis 223(6):981–984
Strich JR, Tian X, Samour M, King CS, Shlobin O, Reger R, Cohen J, Ahmad K, Brown AW, Khangoora V (2021b) Fostamatinib for the treatment of hospitalized adults with coronavirus disease 2019: a randomized trial. Clin Infect Dis
Strilic B, Yang L, Albarrán-Juárez J, Wachsmuth L, Han K, Müller UC, Pasparakis M, Offermanns S (2016) Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536(7615):215–218
Sun S, Duan Z, Wang X, Chu C, Yang C, Chen F, Wang D, Wang C, Li Q, Ding W (2021) Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis 12(6):606. https://doi.org/10.1038/s41419-021-03896-1
Tadie J-M, Bae H-B, Jiang S, Park DW, Bell CP, Yang H, Pittet J-F, Tracey K, Thannickal VJ, Abraham E (2013) HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol-Lung Cell Mol Physiol 304(5):L342–L349
Takesue S, Ohuchida K, Shinkawa T, Otsubo Y, Matsumoto S, Sagara A, Yonenaga A, Ando Y, Kibe S, Nakayama H (2020) Neutrophil extracellular traps promote liver micrometastasis in pancreatic ductal adenocarcinoma via the activation of cancer-associated fibroblasts. Int J Oncol 56(2):596–605
Tarrant BJ, Snell G, Ivulich S, Button B, Thompson B, Holland A (2019) Dornase alfa during lower respiratory tract infection post-lung transplantation: a randomized controlled trial. Transpl Int 32(6):603–613
Teijeira A, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I (2020) CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity, 52(5), 856–871. e858.
Thiam HR, Wong SL, Qiu R, Kittisopikul M, Vahabikashi A, Goldman AE, Goldman RD, Wagner DD, Waterman CM (2020) NETosis proceeds by cytoskeleton and endomembrane disassembly and PAD4-mediated chromatin decondensation and nuclear envelope rupture. Proc Natl Acad Sci 117(13):7326–7337
Tillack K, Breiden P, Martin R, Sospedra M (2012) T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol 188(7):3150–3159
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A (2016) Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Can Res 76(6):1367–1380
Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5(10):e1000639
Valkenburg KC, de Groot AE, Pienta KJ (2018) Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol 15(6):366–381
van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, Tohme S, Loughran P, O’Doherty RM, Minervini MI (2018) Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 68(4):1347–1360
Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, Rubin CJ, Zhao W, Olsen SH, Klinker M (2011) Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187(1):538–552
von Meijenfeldt FA, Burlage LC, Bos S, Adelmeijer J, Porte RJ, Lisman T (2018) Elevated plasma levels of cell-free DNA during liver transplantation are associated with activation of coagulation. Liver Transpl 24(12):1716–1725
Wang X, Qiu L, Li Z, Wang X-Y, Yi H (2018) Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front Immunol 9:2456
Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V (2015) Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science, 349(6245), 316–320.
Whipple CA, Korc M (2010) Angiogenesis Signaling Pathways as Targets in Cancer Therapy. In Handbook of Cell Signaling (pp. 2895–2905). Elsevier.
Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z (2020) Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 11(1):1–19
Wong CH, Jenne CN, Petri B, Chrobok NL, Kubes P (2013) Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 14(8):785–792
Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, Wang Y, Yang S, Liang C, Liang Y (2021) Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell, 39(3), 423–437. e427.
Yang X, Li L, Liu J, Lv B, Chen F (2016) Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-κB and AP-1. Thromb Res 137:211–218
Yang L, Liu Q, Zhang X, Liu X, Zhou B, Chen J, Huang D, Li J, Li H, Chen F (2020a) DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583(7814):133–138
Yang L-Y, Luo Q, Lu L, Zhu W-W, Sun H-T, Wei R, Lin Z-F, Wang X-Y, Wang C-Q, Lu M (2020b) Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol 13(1):1–15
Yazdani HO, Roy E, Comerci AJ, van der Windt DJ, Zhang H, Huang H, Loughran P, Shiva S, Geller DA, Bartlett DL (2019) Neutrophil extracellular traps drive mitochondrial homeostasis in tumors to augment growth. Can Res 79(21):5626–5639
Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 18(9):1386–1393
Yuan K, Zheng J, Huang X, Zhang Y, Han Y, Hu R, Jin X (2020) Neutrophil extracellular traps promote corneal neovascularization-induced by alkali burn. Int Immunopharmacol 88:106902
Zenlander R, Havervall S, Magnusson M, Engstrand J, Ågren A, Thålin C, Stål P (2021) Neutrophil extracellular traps in patients with liver cirrhosis and hepatocellular carcinoma. Sci Rep 11(1):1–11
Zha C, Meng X, Li L, Mi S, Qian D, Li Z, Wu P, Hu S, Zhao S, Cai J (2020) Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL-8 axis. Cancer Biol Med 17(1):154
Zhang H, Goswami J, Varley P, van der Windt DJ, Ren J, Loughran P, Yazdani H, Neal MD, Simmons RL, Zhang J (2020a) Hepatic surgical stress promotes systemic immunothrombosis that results in distant organ injury. Front Immunol 11:987
Zhang S, Jia X, Zhang Q, Zhang L, Yang J, Hu C, Shi J, Jiang X, Lu J, Shen H (2020b) Neutrophil extracellular traps activate lung fibroblast to induce polymyositis-related interstitial lung diseases via TLR9-miR-7-Smad2 pathway. J Cell Mol Med 24(2):1658–1669
Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, Zoltan M, Arora N, Baydogan S, Horne W (2020c) Interleukin-17–induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med 217(12):e20190354
Zhang H, Wang Y, Onuma A, He J, Wang H, Xia Y, Lal R, Cheng X, Kasumova G, Hu Z (2021) Neutrophils Extracellular Traps Inhibition Improves PD-1 Blockade Immunotherapy in Colorectal Cancer. Cancers 13(21):5333
Zhou P, Li T, Jin J, Liu Y, Li B, Sun Q, Tian J, Zhao H, Liu Z, Ma S (2020) Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicine 53:102671
Zhu T, Zou X, Yang C, Li L, Wang B, Li R, Li H, Xu Z, Huang D, Wu Q (2021) Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelial-mesenchymal transition. Int J Mol Med 48(1):1–13
Funding
Open Access funding enabled and organized by Projekt DEAL. Wenxing Hu is supported by a scholarship grant from Chinese Scholarship Council. The other authors do not receive any financial support for the research, authorship and publication of this article.
Author information
Authors and Affiliations
Contributions
All authors reviewed the literature. Wenxing Hu drafted the manuscript. Serene M.L. Lee, Hanno Nieß and Alexandr V. Bazhin revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All the authors have no relevant conflict interests to disclose.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, W., Lee, S.M.L., Bazhin, A.V. et al. Neutrophil extracellular traps facilitate cancer metastasis: cellular mechanisms and therapeutic strategies. J Cancer Res Clin Oncol 149, 2191–2210 (2023). https://doi.org/10.1007/s00432-022-04310-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04310-9