Abstract
Purpose
Oligometastatic prostate cancer is heavily investigated, and conventionally fractionated elective nodal treatment appears to increase biochemical relapse-free (bRFS) survival. The novelty of this report is to present elective nodal radiotherapy (ENRT) with simultaneous integrated boost with stereotactic (SBRT) or hypofractionated radiotherapy (HoFRT) for tolerance and for bRFS which we compared with SBRT of the involved field (IF) only.
Materials and methods
Patients between 2018 and 2021 with and oligometastatic prostate cancer treated with SBRT or hypofractionation were eligible. A radiobiologically calculated simultaneous integrated boost approach enabled to encompass elective nodal radiotherapy (ENRT) with high doses to PSMA-positive nodes. A second group had only involved field (IF) nodal SBRT.
Results
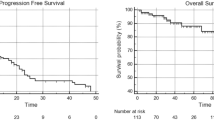
A total of 44 patients with 80 lesions of initially intermediate- (52%) or high-risk (48%) D’Amico omPC were treated with SBRT to all visible PSMA-PET/CT lesions and 100% of the treated lesions were locally controlled after a median follow-up was 18 months (range 3–42 months). Most lesions (56/80; 70%) were nodal and the remainder osseous. Median bPFS was 16 months and ADT-free bPFS 18 months. ENRT (31 patients) versus IF (13 patients) prevented regional relapse more successfully. At univariate analysis, both initial PSA and length of the interval between primary diagnosis and biochemical failure were significant for biochemical control. Treatment was well tolerated and only two patients had toxicity ≥ grade 3 (1 GU and 1 GI, each).
Discussion/conclusion
SBRT and hypofractionated radiotherapy at curative doses with ENRT was more effective to delay ADT than IF, controlled all treated lesions and was well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oligometastatic prostate cancer is a catch-all word that refers to at least two separate entities with probable unique biochemical signatures and behaviors. Oligorecurrent prostate cancer refers to the development of limited distant sites of dissemination following primary radical prostatectomy (RP) or radiotherapy (RT), whereas de novo oligometastasis refers to a distinct group of patients with prostate cancer that has spread to limited areas prior to receiving definitive therapy. As a result, viable treatment methods for de novo oligometastases must take the complete source tumor into account in addition to distant lesions. A third form, dubbed oligoprogression, is also developing. It refers to individuals who have extensive metastases but have just a few sites of progression on systemic treatment (Cheung 2016; Franzese et al. 2018; Triggiani et al. 2017). Notably, oligoprogressive illness was underrepresented in retrospective investigations, despite the fact that series with mixed histologies (Pembroke et al. 2018), and prostate cancer in particular (Franzese et al. 2018; Triggiani et al. 2017), have revealed a poorer prognosis for individuals with typical oligometastasis.
Typically, individuals with oligometastatic illness were diagnosed using a specified criteria for the number of distant locations implicated. Recent research, however, indicates that individuals with oligometastases have a better prognosis. Tree et al. postulated that treating oligometastases might increase PFS (progression-free survival) by 2–4 years in 20%–40% of patients with various types of cancer, including prostate carcinoma (Tree et al. 2013). There is currently no agreement on the number of nodal or bone lesions that define the "oligo" stage except the ESTRO-ASTRO consensus (Lievens et al. 2020), Lievens and colleagues defined the Oligometastic disease (OMD) can to date be defined as 1–5 metastatic lesions, a controlled primary tumor being optional, but where all metastatic sites must be safely treatable.
Feasibility findings on metastasis-directed treatment for oligorecurrences without ADT indicate that S2018BRT (stereotactic body radiation) has a low toxicity rate (Ost et al. 2016; Kneebone et al. 2018; Siva et al. ). Prospective findings using choline or sodium fluoride PET (Positron Emission Tomography) SBRT (Ost et al. 2016; Kneebone et al. 2018) suggest an increase in PFS and a delay in the requirement for ADT when compared to observation alone (Ost et al. 2018). However, in the PSMA-PET era, Kneebone et al. demonstrated conflicting results, with distant recurrences occurring in the majority of patients within 15 months of follow-up (Kneebone et al. 2018). Considering this controversy regarding the impact of local therapy in limited stage IV disease, we chose to conduct a retrospective analysis of patients who received ablative RT (radiotherapy) for < five macroscopic oligometastases (rcN1/cM1).
The purpose of this research was to determine the effectiveness and safety of stereotactic body radiotherapy (SBRT/IGRT) in patients with oligometastatic disease who had a biochemical recurrence following prior surgery. We are the first to describe a combined approach of ENRT with SIB using non-conventional single doses but exclusively SBRT or a hypofractionated fractionation schedule.
Materials and methods
Patient selection
Patients treated between March 2018 and October 2021 at the Department of Radiation Oncology at the University Hospital Magdeburg in Germany with SBRT or hypofractionated radiotherapy of ≥ 3 Gy per fraction for oligometastatic lymph nodes or bone metastases were included in this retrospective analysis. Only patients with less than or equal to five metastases at the time of SBRT were included in the study. Many published studies including an international clinical trial (Lievens et al. 2020; Palma et al. 2012; Milano et al. 2008) have defined oligometastatic disease as having less than or equal to five metastases, and this criterion was utilized in our analysis. Based on the PSMA-PET/CT scan, the diagnosis of oligometastatic illness was confirmed (Fig. 1).
Patients were included in the study if they were above the age of 18, and if they had an Eastern Cooperative Oncology Group (ECOG) score of 2 or less. Repeated SBRT to the same location or verified cerebral metastases were exclusion criteria from the study. SBRT was confirmed and agreed choice of therapy in all cases that were assessed by a multidisciplinary board. The institutional ethics committee gave its support to this study.
Treatment and follow-up
Patients were immobilized with a vacuum bag and scanned with their arms raised in a supine posture. For treatment of the upper thoracic and cervical areas, additional thermoplastic upper body or head and neck masks were employed. There were no fiducials used. All subjects in the simulation underwent a conventional CT scan with IV contrast and oral contrast if indicated. The treating radiation oncologist decided whether to do an extra 4DCT scan on lymph nodes that were located in position that could not be excluded to be moving with respiration during treatment. Baseline diagnostic PSMA-PET/CT was either performed in planning position or they were fused with planning CT images to the region of interest for tumor delineation. CT simulation and/or PSMA-PET/CT scans were used to determine the gross tumor volume (GTV). No additional CTV margins were added to the GTV. The respective GTVs shown on all phases of the 4DCT scan were aggregated to form an internal target volume (ITV) in circumstances when 4DCT simulation was used. An isotropic margin of 4 mm (mm) was added to the ITV to produce the planning target volume (PTV). An isotropic margin of 4 mm on the CTV was utilized to determine the PTV for patients who did not get a 4DCT simulation scan and for CTVs of elective nodal regions as well as vertebral bodies. IMRT plans were used for all patients.
The Ray-Station™ (Stockholm, Sweden) software and TomoTherapy (Accuray, Sunnyvale, CA, USA) software were used to optimize treatment programs. Different SBRT regimens were utilized depending on the target dimension or metastases location. When dose restrictions for organs at risk were not satisfactory, either replanning or a more protracted fractionation schedule was considered. Generally, hypofractionation and not SBRT was chosen if OAR constraints could not be respected with SBRT and if the OARs of the prostatic fossa were part of the treatment volume. Using daily CBCT, all the patients underwent daily IGRT to ensure the delivery of the right doses to the target volume and to avoid the overdose for OAR.
After radiation therapy, patients were followed up by radiation oncologists every 2–4 months. PSMA-PET/CT, MRI, or CT were all used for routine follow-up imaging.
The local tumor response was evaluated using RECIST (Endpoints Response Evaluation Criteria in Solid Tumors; Eisenhauer et al. 2009). The primary endpoints for oligorecurrent PC were distant progression-free survival (DPFS, defined as the time from the first day of SBRT to the detection of clinical disease outside the PTV following further biochemical progression) and ADT-free survival (ADT-FS, defined as the time from the first day of SBRT to the start of palliative ADT). The main endpoints in oligo-CRPC were DPFS and second-line systemic treatment-free survival (STFS, defined as the time between the first day of SBRT to the start of systemic therapies such as Abiraterone, Enzalutamide, or Docetaxel). The Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 were used to grade the toxicity of radiation measured from the final day of SBRT and censored at the time of disease development or subsequent treatment. Local failure, regional failure, or metastatic progression are all examples of progression. The OS endpoint was failure if a patient died before the end of follow-up, regardless of the reason of death or the date of the final follow-up.
Statistical analysis
Quantitative factors were reported using the median and range, and qualitative variables were presented using frequency and percentage. SPSS 20.0 software was used for statistical analysis (SPSS for Window, IBM Corp., Armonk, NY, USA). Kaplan–Meier analysis was used to determine actuarial rates of LC, progression-free survival (PFS), and overall survival (OS). The time to event was defined as the interval between the date of diagnosis or SBRT, respectively, and the occurrence of the first clinical or radiological finding suggestive of disease recurrence.
The correlation between patient-related characteristics and treatment outcome was determined using log-rank testing. Pearson correlations between parameters were determined. Statistical significance was defined as a p value of ≤ 0.05.
Results
Characteristics of patients
In terms of 68 Ga-PSMA-PET/CT-directed radical irradiation of all lesions for BCR following surgery or definitive radiotherapy, 44 patients met the inclusion criteria (Table 1). Additionally, following a 68 Ga-PSMA-PET/CT-restaging for BCR, six patients underwent another course of SBRT/IGRT for newly diagnosed oligorecurrent metastases and one patient underwent two courses of SBRT.
Overall, 52 treatments (44 patients) of 80 oligometastases (total of 56 lymph-node metastases and 24 bone metastases) were conducted, together with one simultaneously discovered local recurrence (LR) (80 lesions). Solitary metastases were found in 19 patients (43%). (Table 1 patients’ characters). SBRT, defined as by Guckenberger et al. (2020), in 1–12 fractions was employed in 69% of the patients and hypofractionation in 31% (Table 2) (Figs. 2, 3).
The median age at the time of the initial diagnosis was 64.4 years (range 52–76 years) and the mean ECOG performance status was 0 (range: 0–2). Regarding pTNM, the majority of patients had at least one high-risk factor (pT3a: 15; pT3b: 13; pN1: 7; R1:10. Twenty-seven patients (61%) had a history of previous irradiation of the prostatic fossa in salvage or adjuvant settings (total dose: 66.0–74.0 Gy) 27.8 months (median; range 6–48 months) following RPVE.
All patients had 68 Ga-PSMA-PET/CT imaging for BCR, which was triggered by at least two PSA spikes of 0.2 ng/mL or above. At the time of imaging, the average PSA level was 1.8 ng/mL (range: 0.23– 33). Eighty lesions positive for 68 Ga-PSMA were discovered in PET/CT at a mean of 66 months (range 5–228 months) after RPVE. The majority of nodal oligometastases were found in the iliac and perirectal regions. The majority of bone metastases were seen in the pelvic bones.
Follow-up was 21 months on average (range, 3–42) and with a median of 19. Prior to SBRT, the median PSA level was 1.82 ng/dL, mean 4,067 (range, 0.23–33).
Mean size of the PSMA-GTV was 4.8 mL (median 3.8; range: 0.22–20.69) and mean PTV was 27.8 mL (median: 25; range: 3.3–88). A total of 31 treatments were with SIB-concept adding an elective treatment of the nodal region to an EQD22 of 50 Gy.
Mean dose was 44.75 Gy (median 42; range 35–60) in a mean of 11.7 fractions (median, 12 range 5–20), with a mean daily dose of 4.37 Gy (median 4; range 3–8 Gy). Mean BED2 was 144.62 Gy (range 140–150), mean EQD23 was 68.42 Gy, and mean EQD210 was 56.923 Gy, while mean EQD22 was 72,3.
Outcome
In terms of effectiveness, the median PSA-nadir level following the completion of RT was 0.49 ng/mL. In 44 individuals, biochemical relapse, defined as a rise of PSA after reaching the nadir 3 times in a row or above 2 ng/mL or positive PSMA-PET lesion, occurred after an average of 16 months (range: 3–39) and median of 14 months. At the time of relapse, the median PSA level was 1.89 ng/mL (range: 0.8–11.57) (Figs. 1, 4).
Twenty of the 44 patients achieved biochemical control following the first SBRT, whereas 24 individuals developed PSA recurrence out of field. Following the first SBRT, three of these patients suffered a failure in the prostate bed. These patients had the following characteristics at first diagnosis: 1 intermediate and 2 high-risk D’Amico group, 1 with R1 resection, 1 with Pn1 category, all of them post RPVE and intervals between first therapy and biochemical relapse of 34, 46 and 18 months. None of them was treated with radiotherapy at first diagnosis. Two of them were treated with salvage EBRT and, subsequently, achieved complete PSA control. The third underwent salvage hypofractioned radiotherapy to the PSMA lesion in the prostatic fossa as he experienced a salvage EBRT 3 years prior to biochemical failure (Fig. 5).
Only 4 patients underwent a second course of SBRT, and all of them achieved a second biochemical control. Eleven patients received ADT after a median of 17 months after SBRT (range 6–33 Months). Only 5/44 Patients underwent SBRT under ADT. Three of them experienced biochemical failure after a median of 18 months after SBRT. Two of those who developed biochemical failure received chemotherapy and one patient was a candidate for Lutetium radio-ligand therapy. Three of 44 patients showed biochemical failure, without any PSMA + ve lesions, they underwent wait and see strategy.
Patterns of failure
The following pattern of relapse emerged in patients who suffered a PSA recurrence following SBRT treatment (Table 3). Three patients demonstrated disease progression in the prostatic bed (treated with salvage EBRT). Recurrence in lymph nodes occurred in nine individuals, but only three patients were eligible for SBRT: six patients had recurrent disease and were treated with systemic treatment (all treated with ADT), because they were identified as having disseminated disease (Fig. 6).
No recurrences were observed in lesions that were treated by SBRT or hypofractionated radiotherapy. It is worth noting that three out of nine patients with nodal recurrence and oligometastatic disease relapsed in a node adjacent to previously treated lymph nodes but out of radiation field. Patients with bone metastases treated with SBRT who relapsed did not demonstrate any spread in the immediate vicinity of the treated lesion: they developed new distant metastasis in other bone segments.
Toxicity
All patients received the full course of treatment, which was usually well tolerated with a minor pattern of toxicity. In the acute context, 42/44 individuals experienced Grade 1–2 side effects. The most often reported effects were nausea and fatigue. During follow-up, only two patients reported > grad 3e gastrointestinal (GI)-Toxicity, one suffered from GI grade 3 toxicity, and these resolved with medications. GI grade 4 toxicity occurred in only one patient, who had two lymph nodes (iliacal and pararectal) and who was treated with 12 fractions rather than fewer fractions to reduce bowel toxicity, Despite rectal and colon dose within the constrains; the patient developed a colon perforation. This occurred potentially due to increased bowel sensibility as suggested by a history of spontaneous perforation 2 years before radiotherapy by chronic diverticulosis. Another patient was reported to have grade 3 Genitourinal (GU) toxicity. Geriatric evaluations revealed no deterioration in health status during the length of the follow-up.
Finally, we conducted uni- and multivariate analyses for biochemical control of patients with oligometastatic disease (Table 4). Improved BCR-PFS was not linked to a history of previous radiotherapy of the prostatic fossa, Gleason score, loco-regional radiotherapy, PSA nadir, and age in our analyses using the log-rank statistic and Kaplan–Meier estimates (p = 0.505, 0.903, 0. 602, 0.091, and 0.354, respectively). Although not statistically significant, there was a tendency toward improved survival in patients with a single lesion versus those with more than one lesion (p = 0.405; HR: 0.605); however, Kaplan–Meier demonstrated a superior PFS in the first 2 years (Fig. 7). A decrease in PSA following SBRT was not associated with an improved prognosis for OS. The only two variables associated with improved PFS, and ADT-PFS were baseline PSA prior SBRT and longer intervals between RPVE and the BCF (p = 0.004 and 0.007, HR: 1.010 and 0.752, respectively) (Fig. 8).
Discussion
To the best of our knowledge, this is the first published study to report a technique combining ENRT with a boost to the IF volume using either SBRT or hypofractionation. All other published studies on this combination have used conventional fractionation (CF) schedules (Ost et al. 2016; Bleser et al. 2017; Phillips et al. 2020; Koerber et al. 2020; Fodor et al. 2017; Würschmidt et al. 2011; Lépinoy et al. 2019; Kirste et al. 2021; Decaestecker et al. 2014).
In this retrospective analysis, we describe our experience with patients who received Linac-based SBRT-IMRT for oligometastatic prostate cancer. In our experience, patients with an oligometastatic status who underwent SBRT or ENRT with a boost to the IF volume had excellent local control. Indeed, all the treated lesions were locally controlled in our analysis after 1 and 2 years. This finding corroborates Palma DA et al., indicating that targeted treatment, such as SBRT, is an effective method of disease management in patients with four or fewer metastatic lesions (Palma et al. 2012). Additionally, dose administration using daily cone-beam CT (CBCT) conducted prior to each session of RT ensures adherence to dose restrictions for organs at risk and achievement of prescription to the target, hence limiting the pattern of toxicity. In our cohort, we experienced no relevant differences in the toxicities between the two techniques. Nevertheless, one patient, who underwent ENRT, suffered a grade 4 GI toxicity which was attributed to a previous history of perforation by known diverticulosis. This occurred despite respecting the dose constraints to the OARs. However, it might still serve as a warning sign for the GI tolerance when employing hypofractioned regimes (Fig. 5; Table 5).
In our study, we treated 13 patients using a focal strategy by targeting only the lesion (IFRT), while in 31/44, we used elective radiation technique with SIB (ENRT). The analysis did not show differences in PFS or ADT-free survival. There is a lack of comparative evidence for IF methods vs elective RT. A total of 62 patients with nodal oligorecurrent PC were treated with either elective nodal RT or involved node SBRT and analyzed in a study by Lepinoy et al. for outcome and toxicity (Lépinoy et al. 2019). Twenty-seven patients suffered biochemical failure after a median follow-up of 41.8 months (5.9–108.1 months), with 23 in the IFRT group and 4 in the ENRT group. The median 3-year overall failure rate of 69.5 percent (95 percent CI 56.0–79.6 percent). The median 3-year-freedom from failure was improved with s-EFRT: in the IFRT group, it was 55.3 percent (95 percent CI 37.0–70.3 percent), and in the ENRT group, it was 88.3 percent (95 percent CI 66.9–96.1%; p = 0.0094). Late gastrointestinal and urinary tract toxicities were not significantly different between groups (p = 0.576 for both).
Similar findings of reduced nodal recurrences were made by De Bleser et al. (2017). They observed a significantly higher rate of late toxicities with ENRT compared to IFRT (16% in 130 patients vs 5% in 202 patients) including 3 patients with grade 3 GU and 1 patient with grade 4 combined GI/GU toxicities. Finally, a multicenter report of 204 patients with IF versus 190 patients with ENRT found significantly improved 3-year bPFS in the latter group with 61% versus 22%. Again, higher toxicity was reported with ENRT at 0.8% and 3% for acute and late grade ≥ 3 versus none in the IF group.
Another German study by Kriste et al. showed PSMA directed radiotherapy plus elective RT was associated with a significantly improved 3-year bRFS compared to PSMA directed radiotherapy alone (53 vs. 37%; p = 0.001; Kirste et al. 2021). This research looked primarily at elective RT of the prostate bed. Patients who received just ENRT progressed considerably more often and had a lower 3-year bRFS (22%) than patients who received both ENRT and elective prostate bed RT (ePBRT) (3-year bRFS 61 percent; p 0.001). The majority of patients (52.0%) were treated with conventionally fractionated RT or conventionally fractionated RT with a simultaneous integrated boost (SIB) approach 130. (33.0 percent). SBRT was employed in 38 (9.6%) of the patients, while combination of SBRT and conventional RT was used in 21 (5.4%) of the patients.
These comparisons support the hypothesis that invisible microscopical nodal metastases in the vicinity of the PET-positive nodes lead to recurrent disease. In the light of these studies with CF therapy, the current study agrees with these results with respect to regional control and to moderately increased toxicity, however, with shorter treatment duration for ENRT.
SBRT was effective in controlling tumor regardless of the location of recurrence, with response rates nearly comparable in cases of nodal disease and bone metastases. In patients with nodal disease who relapsed following RT and continued to have an oligometastatic pattern of disease, failure invariably occurred in nodes next to previously treated lymph nodes, similar to what Decaester et al. reported (2014). We hypothesize that this finding is significant, because it may be utilized to alter the SBRT method in patients with nodal metastases by delivering a fractionated radiation dosage to the affected chain's lymph nodes. Because imaging methods identify micrometastatic illness infrequently, the disease burden in the nearest lymph nodes is underestimated (Tilki et al. 2013) (Fig. 2).
Pasqualetti F et al. evaluated the clinical results of 29 patients with oligometastatic PCa treated with [18F] FMCHPET/CT-guided SBRT in prospective research, and there was a substantial influence on patient treatment management (Pasqualetti et al. 2016). It achieved disease control, as measured by PSA levels, in 20 individuals, avoiding the requirement of systemic medication. In the remaining nine patients, he postponed the start of systemic medication for 36 months. For SBRT, it was discovered that the higher the EQD2 (equivalent dose in 2-Gy fractions) was, the better the overall survival rate (BPFS) was determined to be by Schick et al. (HR 0.37, p = 0.034; Zacho et al. 2018).
Ost et al. (2016) investigated the pattern of relapse following SBRT for nodal recurrent PCa. They studied 72 patients treated for 89 lymph-node metastases and discovered additional metastases in 68% of instances. They evaluated 72 patients treated for 89 lymph-node metastases and discovered that 68% of newly metastases occurred outside the RT-Field, with a median distant-PFS of 21 months. According to the authors, 88 percent of patients had more than three metastases at the time of BCR. They reported that 14% of patients treated with s-IFRT experienced grade 1 toxicity, 3% had grade 2 toxicity, and none had grade 3 or greater toxicity in large multicenter retrospective research.
Another German group led by Habl et al. (2017) conducted a retrospective study on a limited number of patients (15 patients) with oligometastatic prostate cancer who had no more than two bone metastases and were treated with curative SBRT (Biological Effective Dose-BED > 100 Gy) to the metastatic sites. They reported that the median b-RFS was 6.9 months (range: 1.1–28.4 months), and the 2-year LRFS was 100%. They demonstrated that curative SBRT for metastatic disease is safe and has a high rate of local control. Similarly, Jereczek-Fossa et al. (2017) used SBRT to treat 94 patients with nodal oligometastatic disease, administering a median total dose of 24 Gy (range 15–36 Gy) in three fractions (median BED 152 Gy). The majority of patients (94.7%) had one or two metastatic nodes.
Our study demonstrates that postoperative RT can eliminate LR (local recurrence). In our cohort, from 17 patients who have no history of irradiation of prostatic fossa, 3 patients (17.6%) developed local prostate bed recurrence after ablative RT for oligometastases. That is also evidenced by the low rate of LR in patients treated with RPVE + RT compared to the RPVE-only group by Sobol et al. (2017). mpMRI and Choline PET were utilized in this work to physically map early patterns in 223 participants with BCR among a particularly pure cohort of post-RP patients who had not received any radiation or ADT treatment. The findings revealed that most varieties of relapse are neither as localized nor as broadly metastatic as often assumed, but rather a mix of local and/or regionally metastatic recurrences contained inside the pelvis. This mapping investigation provided additional evidence for the expansion of salvage RT fields into the pelvis as reviewed in RTOG 0534. Assuming total disease eradication by salvage RT, a cure response might be expected in 33% of men treated with salvage fossa irradiation alone. Extending the sRT area to include pelvic lymph nodes distal to the common iliac bifurcation may increase curative responses closer to 50%.
Also, another study by Rischke et al. provides more evidence for the underestimation of nodal disease in PET/CT (Rischke et al. 2015). Patients in this research received extra RT following PET/CT-guided salvage lymph-node resection. The addition of RT to the areas with PSMA-expressing tumors on PET/CT improved 5-year PFS from 26.3 to 70.7 percent, demonstrating residual micrometastasis following surgery. In a similar manner to nodal illness, underestimating of subclinical, microscopic disease is likely to occur in the prostate bed, which has the highest risk of microscopic disease following radical prostatectomy. In addition to the poor spatial resolution of PET/CT, tracer excretion via the bladder, with subsequent blurring of the region of the prostatic fossa, makes detecting a local recurrence in the prostate fossa challenging. Our data suggest that RT to the prostate bed can be omitted and done as salvage if required to avoid unnecessary GU/GI toxicity.
Five of our 44 participants underwent ADT concurrently. Due to the uncertain timing of ADT initiation within the patient population, the PSA-PFS findings should be evaluated cautiously. In a prospective single-arm Spanish-Swiss research (Schick et al. 2013), the combination of locally ablative RT and concurrent ADT resulted in a PFS of 54%, a clinical PFS of 58 percent, and an OS of 92% after a median time period of 30 months. ADT was not administered in a systematic manner and was administered for a median of 18 months (range, 3–34 months).
Due to the retrospective and monocentric nature of our investigation, there are significant limitations. Additionally, because the median duration of follow-up is relatively short, the essential endpoints of overall survival and prostate cancer-specific survival could not be examined. The majority of patients underwent pretreatment imaging with PSMA-PET/CT, which was proven to have a high sensitivity for identifying prostate cancer metastases (Zacho et al. 2018). However, due to the absence of histologic confirmation, we were unable to rule out false-positive and false-negative PSMA-PET lesions. Concurrent ADT administration was inconsistent, complicating the assessment of PSA kinetics and bPFS.
However, in the absence of further prospective data, this study throws some insight on the hitherto underrepresented population of patients with bone oligometastatic prostate cancer treated with MDT and classified according to current ESTRO/EORTC oligometastatic disease subclassifications. Although hormone sensitivity and the oligometastatic disease category were no longer significant predictors of oncological outcome in the multivariate analysis, the large differences in oncological outcome between the groups underscore the need for a more precise classification of patients with bone oligometastatic disease (Fig. 3).
Conclusion
In conclusion, our findings show that PSMA-PET/CT-guided stereotactic and hypofractionated radiotherapy with elective nodal therapy may be a viable therapeutic option for OMPC patients, including those with recurrent oligometastatic disease. The hypofractioned ENRT in 12 fractions looks to be safe and feasible, and not associated with high toxicity. The beginning or escalation of palliative ADT, as well as its possible adverse effects, can be prevented in this manner. SBRT-treated metastases had high local control rates with low toxicity. Elongated PFS is predicted by low PSA levels. Randomized trials are required to validate our findings.
Abbreviations
- RP:
-
Radical prostatectomy
- RT:
-
Radiotherapy
- SBRT:
-
Stereotactic body radiation therapy
- OMD:
-
Oligometastic disease
- ADT:
-
Androgen deprivation therapy
- ECOG:
-
Eastern Cooperative Oncology Group
- OS:
-
Overall Survival
- PFS:
-
Progression-free survival
- BCF:
-
Biochemical Failure
- IGRT:
-
Image-guided radiation therapy
- PCa:
-
Prostata cancer
- SIB:
-
Simultaneous integrated boost
- HoFRT:
-
Hypofractionated radiotherapy
- bRFS:
-
Biochemical relapse free
- ENRT:
-
Elective nodal radiotherapy
- IF:
-
Involved field
- BCR:
-
Biochemical recurrence
- rcN1:
-
Oligorecurrent nodal disease
- M1:
-
Oligorecurrent metastatic disease
References
Cheung P (2016) Stereotactic body radiotherapy for oligoprogressive cancer [serial online]. Br J Radiol 89:20160251
De Bleser E, Tran PT, Ost P (2017) Radiotherapy as metastasis-directed therapy for oligometastatic prostate cancer. Curr Opin Urol 27(6):587–595. https://doi.org/10.1097/MOU.0000000000000441
Bowden P, See AW, Frydenberg M, Haxhimolla H, Costello AJ, Moon D, Ruljancich P, Grummet J, Crosthwaite A, Pranavan G et al (2020) Fractionated stereotactic body radiotherapy for up to five prostate cancer oligometastases: Interim outcomes of a prospective clinical trial. Int J Cancer 146:161–168
Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T et al (2014) Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 9:135
Deek MP, Taparra K, Phillips R, Velho PI, Gao RW, Deville C, Song DY, Greco S, Carducci M, Eisenberger M, DeWeese TL, Denmeade S, Pienta K, Paller CJ, Antonarakis ES, Olivier KR, Park SS, Tran PT, Stish BJ (2021) Metastasis-directed therapy prolongs efficacy of systemic therapy and improves clinical outcomes in oligoprogressive castration-resistant prostate cancer. Eur Urol Oncol 4(3):447–455. https://doi.org/10.1016/j.euo.2020.05.004
Eisenhauer E, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (version1.1). Eur J Cancer 45:228–247
Fodor A, Berardi G, Fiorino C, Picchio M, Busnardo E, Kirienko M et al (2017) Toxicity and efficacy of salvage carbon 11-choline positron emission tomography/computed tomography-guided radiation therapy in patients with lymph node recurrence of prostate cancer. BJU Int 119(3):406–413. https://doi.org/10.1111/bju.13510
Franzese C, Zucali PA, Di Brina L et al (2018) The efficacy of stereotactic body radiation therapy and impact of systemic treatments in oligometastatic patients from prostate cancer. Cancer Med 7:4379–4386
Guckenberger M, Baus WW, Blanck O, Combs SE, Debus J, Engenhart-Cabillic R, Gauer T, Grosu AL, Schmitt D, Tanadini-Lang S, Moustakis C (2020) Definition and quality requirements for stereotactic radiotherapy: consensus statement from the DEGRO/DGMP Working Group Stereotactic Radiotherapy and Radiosurgery. Strahlenther Onkol 196(5):417–420. https://doi.org/10.1007/s00066-020-01603-1
Habl G et al (2017) Oligometastases from prostate cancer: local treatment with stereotactic body radiotherapy (SBRT). BMC Cancer 17:361. https://doi.org/10.1186/s12885-017-3341-2
Jereczek-Fossa BA et al (2017) Salvage stereotactic body radiotherapy for isolated lymph node recurrent prostate cancer: single institution series of 94 consecutive patients and 124 lymph nodes. Clin Genitourin Cancer 15(4):e623–e632
Kirste S, Kroeze S, Henkenberens C, Schmidt-Hegemann NS, Vogel M, Becker J, Zamboglou C, Burger I, Derlin T, Bartenstein P, Ruf J, la Fougère C, Eiber M, Christiansen H, Combs SE, Müller AC, Belka C, Guckenberger M, Grosu AL (2021) Combining 68Ga-PSMA-PET/CT-directed and elective radiation therapy improves outcome in oligorecurrent prostate cancer: a retrospective multicenter study. Front Oncol 11:640467. https://doi.org/10.3389/fonc.2021.640467
Kneebone A, Hruby G, Ainsworth H et al (2018) Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol 1(6):531–537
Koerber SA, Katharina S, Clemens K, Erik W, Matthias FH, Sonja K et al (2020) Clinical outcome of PSMA-guided radiotherapy for patients with oligor-ecurrent prostate cancer. Eur J Nucl Med Mol Imaging 48(1):143–151
Lépinoy A, Silva YE, Martin E, Bertaut A, Quivrin M, Aubignac L et al (2019) Salvage extended field or involved field nodal irradiation in 18F-fluorocholine PET/CT oligorecurrent nodal failures from prostate cancer. Eur J Nucl Med Mol Imaging 46(1):40–48. https://doi.org/10.1007/s00259-018-4159-0
Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, Méndez Romero A, Nevens D, Palma D, Park C, Ricardi U, Scorsetti M, Yu J, Woodward WA (2020) Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother Oncol 148:157–166. https://doi.org/10.1016/j.radonc.2020.04.003 (Epub 2020 Apr 22 PMID: 32388150)
Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, Okunieff P (2008) A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 112:650–658
Ost P, Jereczek-Fossa BA, As NV et al (2016) Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol 69(1):9–12
Ost P, Reynders D, Decaestecker K et al (2018) Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. JCO 36(5):446–453
Palma DA, Haasbeek CJ, Rodrigues GB et al (2012) Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABRCOMET): study protocol for a randomized phase II trial. BMC Cancer 12:305. https://doi.org/10.1186/1471-2407-12-305
Pasqualetti F, Panichi M, Sainato A, Matteucci F, Galli L, Cocuzza P et al (2016) [(18)F]Choline PET/CT and stereotactic body radiotherapy on treatment decision making of oligometastatic prostate cancer patients: preliminary results. Radiat Oncol 11:9. https://doi.org/10.1186/s13014-016-0586-x
Pembroke CA, Fortin B, Kopek N (2018) Comparison of survival and prognosis factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiother Oncol 127:493–500
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES et al (2020) Out- comes of observation vs stereotactic ablative radiation for oligometa-static prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol 6(5):650–659
Rischke HC, Schultze-Seemann W, Wieser G, Krönig M, Drendel V, Stegmaier P et al (2015) Adjuvant radiotherapy after salvage lymph node dissection because of nodal relapse of prostate cancer versus salvage lymph node dissection only. Strahlenther Onkol 191(4):310–320. https://doi.org/10.1007/s00066-014-0763-5
Schick U, Jorcano S, Nouet P, Rouzaud M, Vees H, Zilli T, Ratib O, Weber DC, Miralbell R (2013) Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol 52(8):1622–1628
Siva S, Bressel M, Murphy DG et al (2018) Stereotactic Abative Body Radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol 74(4):455–462
Sobol I, Zaid HB, Haloi R et al (2017) Contemporary mapping of post-prostatectomy prostate cancer relapse with 11C-choline positron emission tomography and multiparametric magnetic resonance imaging. J Urol 197:129–134
Tilki D, Reich O, Graser A, Hacker M, Silchinger J, Becker AJ et al (2013) 18F-Fluoroethylcholine PET/CT identifies lymph node metastasis in patients with prostate-specific antigen failure after radical prostatectomy but underestimates its extent. Eur Urol 63(5):792–796 (Evaluation Studies)
Tree AC, Khoo VS, Eeles RA et al (2013) Stereotactic body radiotherapy for oligometastases. Lancet Oncol 14(1):e28–e37
Triggiani L, Alongi F, Buglion M et al (2017) Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer 116:1520–1525
Würschmidt F, Petersen C, Wahl A, Dahle J, Kretschmer M (2011) [18F] fluoroethylcholine-PET/CT imaging for radiation treatment planning of recurrent and primary prostate cancer with dose escalation to PET/CTpositive lymph nodes. Radiat Oncol 6:44. https://doi.org/10.1186/1748-717X-6-44
Zacho HD, Nielsen JB, Haberkorn U, Stenholt L, Petersen LJ (2018) 68Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: a systematic review of the published literature. Clin Physiol Funct Imaging 38(6):911–922. https://doi.org/10.1111/cpf.12480
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors participated in patient treatment and were involved in the preparation of the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gawish, A., Walke, M., Röllich, B. et al. Nodal and osseous oligometastatic prostate cancer: a cohort including the introduction of PSMA-PET/CT-guided stereotactic and hypofractionated radiotherapy with elective nodal therapy. J Cancer Res Clin Oncol 149, 3937–3949 (2023). https://doi.org/10.1007/s00432-022-04229-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04229-1