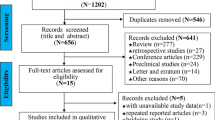
Abstract
Purpose
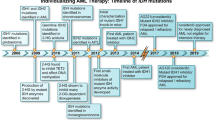
Isocitrate dehydrogenase enzyme 1 (IDH1) mutations at 132nd amino acid residue (R132*) result in the cellular accumulation of the oncometabolite, 2-hydroxyglutarate (2-HG). IDH305 is an orally bioavailable, brain-penetrant, mutant-selective allosteric IDH1 inhibitor demonstrating target engagement in preclinical models. This first-in human study was designed to identify the recommended dose for expansion/maximum tolerated dose of IDH305 in patients with IDH1R132-mutant acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).
Methods
IDH305 was given at doses 75–750 mg twice daily in 41 patients with IDH1R132-mutant AML/MDS. Dose escalation was designed using Bayesian hierarchical model with overdose control principle and relationship with dose-limiting toxicity.
Results
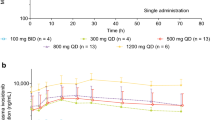
IDH305 exhibited rapid absorption with mean T1/2 approximately 4–10 h across doses. Interpatient variability was moderate and exposure increased with dose in a less than dose proportional manner. Most patients (35/41) demonstrated target engagement with reduction in 2-HG concentration at all doses. Complete remission (CR) or CR with incomplete count recovery occurred in 10/37 (27%) patients with AML and 1/ 4 patients with MDS. Adverse events (AEs) suspected to be related to study drug were reported in 53.7% of patients: increased blood bilirubin (14.6%), nausea (14.6%), increased alanine aminotransferase and aspartate aminotransferase (12.2%, each); most frequent grade 3 or 4 AEs were differentiation syndrome and tumor lysis syndrome (n = 3; 7.3%, each). Hepatotoxicity was manageable with dose modification.
Conclusion
Due to potentially narrow therapeutic window, the study was prematurely halted and recommended phase 2 dose could not be declared.
Trial registration
Clinicaltrials.gov identifier: NCT02381886.





Similar content being viewed by others
Data availability
Novartis will not provide access to patient-level data if there is a reasonable likelihood that individual patients could be reidentified. Phase 1 studies, by their nature, present a high risk of patient reidentification; therefore, patients’ individual results for phase 1 studies cannot be shared. In addition, clinical data, in some cases, have been collected subject to contractual or consent provisions that prohibit transfer to third parties. Such restrictions may preclude granting access under these provisions. Where co-development agreements or other legal restrictions prevent companies from sharing particular data, companies will work with qualified requestors to provide summary information where possible.
References
Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, Pollock R, O’Donnell P, Grigoriadis A, Diss T, Eskandarpour M, Presneau N, Hogendoorn PC, Futreal A, Tirabosco R, Flanagan AM (2011) IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 224(3):334–343. https://doi.org/10.1002/path.2913
de Botton S, Pollyea DA, Stein EM, DiNardo C, Fathi AT, Roboz GJ et al. (2015) Clinical safety and activity of AG-120, a first-in-class potent inhibitor of the IDH1 mutant protein, in a phase I study of patients with advanced IDH1-mutant hematologic malignancies. Poster presented at the 20th Annual Congress of the European Hematology Association, 11–14 June 2015, Vienna, Austria 100704: P563.
Cairns RA, Mak TW (2013) Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov 3(7):730–741
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD, International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia (2003) Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21(24):4642–4649
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Gore SD, Schiffer CA, Kantarjian H (2006) Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood 108(2):419–425
Cho YS, Levell JR, Liu G, Caferro T, Sutton J, Shafer CM, Costales A, Manning JR, Zhao Q, Sendzik M, Shultz M, Chenail G, Dooley J, Villalba B, Farsidjani A, Chen J, Kulathila R, Xie X, Dodd S, Gould T, Liang G, Heimbach T, Slocum K, Firestone B, Pu M, Pagliarini R, Growney JD (2017) Discovery and evaluation of clinical candidate IDH305, a brain penetrant mutant IDH1 inhibitor. ACS Med Chem Lett 8(10):1116–1121
Cortes JE, Watts J, Prebet T, Schiller GJ, Lee S, Yang J et al (2018) FT-2102, an IDH1m inhibitor, in combination with azacitidine in patients with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS): results from a phase I study. Blood 132(suppl 1):1452
Dang L, Jin S, Su SM (2010) IDH mutations in glioma and acute myeloid leukemia. Trends Mol Med 16(9):387–397. https://doi.org/10.1016/j.molmed.2010.07.002
DiNardo CD, Propert KJ, Loren AW, Paietta E, Sun Z, Levine RL et al (2013) Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood 121(24):4917–4924
DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, Routbort M, Patel KP, Brandt M, Pierce S, Garcia-Manero G, Cortes J, Kantarjian H (2015a) Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol 90(8):732–736. https://doi.org/10.1002/ajh.24072
DiNardo CD, Stein EM, Altman JK, Collin R, DeAngelo DJ, Fathi AT et al (2015b) AG-221, an oral, selective, first-in class, potent inhibitor of the IDH2 mutant enzyme, induced durable responses in a phase I study of IDH2 mutation-positive advanced hematologic malignancies. Poster presented at the 20th Annual Congress of the European Hematology Association, 11–14 June 2015b, Vienna, Austria 100710: P569.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, Swords R, Collins RH, Mannis GN, Pollyea DA, Donnellan W, Fathi AT, Pigneux A, Erba HP, Prince GT, Stein AS, Uy GL, Foran JM, Traer E, Stuart RK, Arellano ML, Slack JL, Sekeres MA, Willekens C, Choe S, Wang H, Zhang V, Yen KE, Kapsalis SM, Yang H, Dai D, Fan B, Goldwasser M, Liu H, Agresta S, Wu B, Attar EC, Tallman MS, Stone RM, Kantarjian HM (2018) Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 378(25):2386–2398
Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B, Bloomfield CD, LeukemiaNet E (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474
Fathi AT, Sadrzadeh H, Comander AH, Higgins MJ, Bardia A, Perry A, Burke M, Silver R, Matulis CR, Straley KS, Yen KE, Agresta S, Kim H, Schenkein DP, Borger DR (2014) Isocitrate dehydrogenase 1 (IDH1) mutation in breast adenocarcinoma is associated with elevated levels of serum and urine 2-hydroxyglutarate. Oncologist 19(6):602–607. https://doi.org/10.1634/theoncologist.2013-0417
Fathi AT, DiNardo CD, Kline I, Kenvin L, Gupta I, Attar EC, Stein EM, de Botton S (2018) Differentiation syndrome associated with enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2: analysis of a phase 1/2 study. JAMA Oncol 4(8):1106–1110. https://doi.org/10.1001/jamaoncol.2017.4695
Foran JM, DiNardo CD, Watts JM, Stein EM, DeBotton S, Fathi AT et al (2019) Ivosidenib (AG-120) in patients with IDH1-mutant relapsed/refractory myelodysplastic syndrome: updated enrollment of a phase 1 dose escalation and expansion study. Blood 134(Supplement 1):4254
Fujii T, Khawaja MR, DiNardo CD, Atkins JT, Janku F (2016) Targeting isocitrate dehydrogenase (IDH) in cancer. Discov Med 21(117):373–380
Kosmider O, Gelsi-Boyer V, Slama L, Dreyfus F, Beyne-Rauzy O, Quesnel B, Hunault-Berger M, Slama B, Vey N, Lacombe C, Solary E, Birnbaum D, Bernard OA, Fontenay M (2010) Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia 24(5):1094–1096
Medeiros BC, Fathi AT, DiNardo CD, Pollyea DA, Chan SM, Swords R (2017) Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia 31(2):272–281
Meille C, Parikh N, Ji Y (2020) Mechanism-based target-engagement and safety biomarker pharmacokinetic/pharmacodynamic modeling for IDH305 in early oncology clinical development. QSP-016. American society for clinical pharmacology and therapeutics. Clin Pharmacol Ther 107(1):5–121
Norsworthy KJ, Mulkey F, Scott EC, Ward AF, Przepiorka D, Charlab R, Dorff SE, Deisseroth A, Kazandjian D, Sridhara R, Beaver JA, Farrell AT, de Claro RA, Pazdur R (2020) Differentiation syndrome with ivosidenib and enasidenib treatment in patients with relapsed or refractory IDH-mutated AML: a U.S. food and drug administration systematic analysis. Clin Cancer Res 26(16):4280–4288
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812. https://doi.org/10.1126/science.1164382
Patnaik MM, Hanson CA, Hodnefield JM, Lasho TL, Finke CM, Knudson RA, Ketterling RP, Pardanani A, Tefferi A (2012) Differential prognostic effect of IDH1 versus IDH2 mutations in myelodysplastic syndromes: a mayo clinic study of 277 patients. Leukemia 26(1):101–105. https://doi.org/10.1038/leu.2011.298
Popovici-Muller J, Lemieux RM, Artin E, Saunders JO, Salituro FG, Travins J, Cianchetta G, Cai Z, Zhou D, Cui D, Chen P, Straley K, Tobin E, Wang F, David MD, Penard-Lacronique V, Quivoron C, Saada V, de Botton S, Gross S, Dang L, Yang H, Utley L, Chen Y, Kim H, Jin S, Gu Z, Yao G, Luo Z, Lv X, Fang C, Yan L, Olaharski A, Silverman L, Biller S, Su SM, Yen K (2018) Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett 9(4):300–305. https://doi.org/10.1021/acsmedchemlett.7b00421
Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, Kunii K, Pedraza A, Schalm S, Silverman L, Miller A, Wang F, Yang H, Chen Y, Kernytsky A, Rosenblum MK, Liu W, Biller SA, Su SM, Brennan CW, Chan TA, Graeber TG, Yen KE, Mellinghoff IK (2013) An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340(6132):626–630. https://doi.org/10.1126/science.1236062
Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H, Miltiadous O, Walesky C, Deshpande V, Zhu AX, Hezel AF, Yen KE, Straley KS, Travins J, Popovici-Muller J, Gliser C, Ferrone CR, Apte U, Llovet JM, Wong KK, Ramaswamy S, Bardeesy N (2014) Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature 513(7516):110–114. https://doi.org/10.1038/nature13441
Stein EM, Fathi AT, DiNardo CD, Pollyea DA, Swords RT, Roboz GJ et al (2016) Enadidenib (AG-221), a potent oral inhibitor of mutant isocitrate dehydrogenase 2 (IDH2) enzyme, induces hematologic responses in patients with myelodysplastic syndromes (MDS). Blood 128(22):343
Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, Stone RM, DeAngelo DJ, Levine RL, Flinn IW, Kantarjian HM, Collins R, Patel MR, Frankel AE, Stein A, Sekeres MA, Swords RT, Medeiros BC, Willekens C, Vyas P, Tosolini A, Xu Q, Knight RD, Yen KE, Agresta S, de Botton S, Tallman MS (2017) Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130(6):722–731
Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, Roboz GJ, Patel MR, Collins R, Flinn IW, Sekeres MA, Stein AS, Kantarjian HM, Levine RL, Vyas P, MacBeth KJ, Tosolini A, VanOostendorp J, Xu Q, Gupta I, Lila T, Risueno A, Yen KE, Wu B, Attar EC, Tallman MS, de Botton S (2019) Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood 133(7):676–687
Watts JM, Baer MR, Yang J, Prebet T, Lee S, Schiller GJ, Dinner S et al (2019) Olutasidenib (FT-2102), an IDH1m inhibitor as a single agent or in combination with azacitidine, induces deep clinical responses with mutation clearance in patients with acute myeloid leukemia treated in a phase I dose escalation and expansion study. Blood 134(suppl 1):231
Xu S, Ji Y, Parikh NS (2019) Exposure-response analysis for the efficacy and safety of IDH305, a novel IDH1 inhibitor, in advanced cancer patients. Presented at the 10th American Conference on Pharmacometrics (ACoP10), 20–23 October 2019, Orlando FL 1: T-035 (ISSN: 2688–3953).
Yen K, Travins J, Wang F, David MD, Artin E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin E, Chen Y, Nagaraja R, Choe S, Jin L, Konteatis Z, Cianchetta G, Saunders JO, Salituro FG, Quivoron C, Opolon P, Bawa O, Saada V, Paci A, Broutin S, Bernard OA, de Botton S, Marteyn BS, Pilichowska M, Xu Y, Fang C, Jiang F, Wei W, Jin S, Silverman L, Liu W, Yang H, Dang L, Dorsch M, Penard-Lacronique V, Biller SA, Su SM (2017) AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov 7(5):478–493
Acknowledgements
The authors would like to thank the patients who participated in the trial and their families, and the staff at each site who assisted with the study. Medical writing assistance was provided by Kavita Garg, PhD, CMPPTM of Novartis Healthcare Private Limited.
Funding
This study was sponsored by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Contributions
Conception and design: CDD and NSP. Development of methodology: XC, YJ, NSP, and JC. Acquisition of data: AH, CDD, KY, and AHW. Analysis and interpretation of data: YJ, CDD, AHW, XC, YJ, NSP, and JC. Writing, review, and/or revision of the manuscript: CDD, AH, MGF, KY, TZ, AK, XC, YJ, NSP, JC, and AHW. Administrative, technical, or material support: NSP and JC. Study supervision: JC and NSP.
Corresponding author
Ethics declarations
Conflict of interest
CDD: CDD received research funding from AbbVie, Agios, Calithera, Cleave, BMS/Celgene, Daiichi-Sankyo, Forma, ImmuneOnc, Loxo, and received consultancy or advisory board fees from AbbVie, Agios, Aprea, Celgene/BMS, ImmuneOnc, Novartis, and Takeda. AH: research support from BMS, Incyte, Novartis, Pfizer. MGF: research support from Abbvie and Tolero Pharmaceuticals; current employment and equity ownership at Cellectis Inc. NY. KY: research funding from Agensys, Astex, Forma Therapeutics, Genentech, Jazz, Medimmune, Novartis, Onconova, Roche, and Honoraria from Celgene/BMS, Novartis, Otsuka Pfizer, TaiHo, Takeda. TZ: other support from BMS, MSD, Novartis, Roche. AK: Grants from Celgene, Daiichi Sankyo, Roche, and personal fees from Abbvie, BMS, Daiichi Sankyo, Roche. XC: employee of Novartis. YJ: employee of Novartis. NSP: employee of Novartis at time of study conduct and manuscript writing. JC: employee of Novartis. AHW: honoraria from Novartis, Astellas, Pfizer, Macrogenics, AbbVie, Genentech, Servier, Celgene, Amgen, Astra Zeneca and Janssen; research funding from Novartis, Celgene, AbbVie, Servier, Astra Zeneca and Amgen; former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax.
Ethical approval
The study protocol was approved by the independent ethics committee or institutional review board for each center. The study was conducted according to the principles of the Declaration of Helsinki, and performed in compliance with Good Clinical Practice.
Consent to participate
All the patients provided written informed consent before participation in the clinical trial.
Consent to publication
All the authors have read and approved the final manuscript for submission to “Journal of Cancer Research and Clinical Oncology”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DiNardo, C.D., Hochhaus, A., Frattini, M.G. et al. A phase 1 study of IDH305 in patients with IDH1R132-mutant acute myeloid leukemia or myelodysplastic syndrome. J Cancer Res Clin Oncol 149, 1145–1158 (2023). https://doi.org/10.1007/s00432-022-03983-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03983-6