Abstract
Purpose
Liver metastasis (LM) is common in non-small cell lung cancer (NSCLC), and always predicted worse outcomes with no effective therapy. We aimed to evaluate the effects and prognosis in LM patients treated with anlotinib.
Methods
The present study is a post hoc analysis based on a multicenter, double-blind, phase 3 randomized clinical trial which designed to evaluate the efficacy and safety of anlotinib in patients with advanced NSCLC. A total of 437 patients were enrolled in present study, and 78 patients with LM.
Results
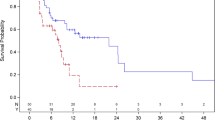
Patients with LM showed a worse outcome compared to those without LM (PFS median, 2.6 vs 4.2 months), and OS (median, 5.6 vs 9.4 months, both P < 0.0001). The anlotinib was associated with longer PFS (median, 3.0 months) compared with placebo (median, 0.9 months), with a hazard ratio (HR) of 0.23 (95%CI, 0.12–0.42; P < 0.0001). Furthermore, OS was marginally significantly better in anlotinib group (median 6.6 months), compared with placebo (median 4.0 months), HR 0.61 (95%CI, 0.36–1.02; P = 0.055). Multivariate analysis confirmed normal peripheral blood LDH/TBiL level predicted better PFS and OS, lower ECOG score acted as independently prognostic factor for superior OS. Anlotinib was more associated with hand–foot syndrome (7.7% vs 0) and serum TSH level rise (7.7% vs 3.8%) and well tolerated, all AEs were no more than grade 3.
Conclusion
Patients with LM had a dismal prognosis, anlotinib could lead to a better PFS in pretreated NSCLC patients, which suggested anlotinib is a potential third-line or further therapy in these patients.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[Chinese Medical Association guidelines for clinical diagnosis and treatment of lung cancer (2019 edition)] (2020) Zhonghua Zhong Liu Za Zhi 42(4):257–287
Castanon E, Rolfo C, Vinal D et al (2015) Impact of epidermal growth factor receptor (EGFR) activating mutations and their targeted treatment in the prognosis of stage IV non-small cell lung cancer (NSCLC) patients harboring liver metastasis. J Transl Med 13:257
Chen F, Chen N, Yu Y, Cui J (2020a) Efficacy and safety of epidermal growth factor receptor (EGFR) inhibitors plus antiangiogenic agents as first-line treatments for patients with advanced EGFR-mutated non-small cell lung cancer: a meta-analysis. Front Oncol 10:904
Chen Z, Wei J, Ma X, Yu J (2020b) Efficacy of EGFR-TKIs with or without angiogenesis inhibitors in advanced non-small-cell lung cancer: a systematic review and meta-analysis. J Cancer 11(3):686–695
Chen Z, Jiang S, Li X et al (2021) Efficacy and safety of anti-angiogenic drugs combined with erlotinib in the treatment of advanced non-small cell lung cancer: a meta-analysis of randomized clinical trials. Ann Palliat Med 10(3):2687–2698
Chu T, Zhong R, Zhong H et al (2021) Phase 1b study of sintilimab plus anlotinib as first-line therapy in patients with advanced NSCLC. J Thorac Oncol 16(4):643–652
Garon EB, Ciuleanu TE, Arrieta O et al (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384(9944):665–673
Han B, Li K, Wang Q et al (2018) Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol 4(11):1569–1575
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hemminki K, Riihimaki M, Sundquist K, Hemminki A (2013) Site-specific survival rates for cancer of unknown primary according to location of metastases. Int J Cancer 133(1):182–189
Hess KR, Varadhachary GR, Taylor SH et al (2006) Metastatic patterns in adenocarcinoma. Cancer Am Cancer Soc 106(7):1624–1633
Hong S, Tan M, Wang S, Luo S, Chen Y, Zhang L (2015) Efficacy and safety of angiogenesis inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 141(5):909–921
Jiang T, Cheng R, Zhang G et al (2017) Characterization of liver metastasis and its effect on targeted therapy in EGFR-mutant NSCLC: a multicenter study. Clin Lung Cancer 18(6):631–639
Jiang S, Liang H, Liu Z et al (2020) The Impact of anlotinib on brain metastases of non-small cell lung cancer: post hoc analysis of a phase III randomized control trial (ALTER0303). Oncologist 25(5):e870–e874
Kitadai R, Okuma Y, Hakozaki T, Hosomi Y (2020) The efficacy of immune checkpoint inhibitors in advanced non-small-cell lung cancer with liver metastases. J Cancer Res Clin Oncol 146(3):777–785
Lin B, Song X, Yang D, Bai D, Yao Y, Lu N (2018) Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene 654:77–86
McKay RR, Kroeger N, Xie W et al (2014) Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol 65(3):577–584
Nakazawa K, Kurishima K, Tamura T et al (2012) Specific organ metastases and survival in small cell lung cancer. Oncol Lett 4(4):617–620
Riihimaki M, Thomsen H, Hemminki A, Sundquist K, Hemminki K (2013) Comparison of survival of patients with metastases from known versus unknown primaries: survival in metastatic cancer. BMC Cancer 13:36
Riihimaki M, Hemminki A, Fallah M et al (2014) Metastatic sites and survival in lung cancer. Lung Cancer 86(1):78–84
Shaw AT, Bauer TM, de Marinis F et al (2020) First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med 383(21):2018–2029
Shiroyama T, Suzuki H, Tamiya M et al (2018) Clinical characteristics of liver metastasis in nivolumab-treated patients with non-small cell lung cancer. Anticancer Res 38(8):4723–4729
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301
Soria JC, Mauguen A, Reck M et al (2013) Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 24(1):20–30
Sun Y, Niu W, Du F et al (2016) Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 9(1):105
Sun D, Li H, Cao M et al (2020) Cancer burden in China: trends, risk factors and prevention. Cancer Biol Med 17(4):879–895
Taurin S, Yang CH, Reyes M et al (2018) Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer 28(1):152–160
Tumeh PC, Hellmann MD, Hamid O et al (2017) Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 5(5):417–424
Vokes EE, Ready N, Felip E et al (2018) Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29(4):959–965
Wang L, Shi T, Feng L et al (2021a) The Prognostic value of baseline distant metastasis in icotinib-treated patients with EGFR-mutated stage IV non-small cell lung cancer. Cancer Manage Res 13:2613–2622
Wang P, Fang X, Yin T, Tian H, Yu J, Teng F (2021b) Efficacy and safety of anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-a retrospective study. Front Oncol 11:628124
Wu KL, Tsai MJ, Yang CJ et al (2015) Liver metastasis predicts poorer prognosis in stage IV lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer 88(2):187–194
Xu H, Ma D, Yang G et al (2019) Sequential therapy according to distinct disease progression patterns in advanced ALK-positive non-small-cell lung cancer after crizotinib treatment. Chin J Cancer Res 31(2):349–356
Yang J, Zhang Y, Sun X et al (2018) The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol 144(9):1835–1842
Zhang K, Ma X, Gao H et al (2020) Efficacy and safety of anlotinib in advanced non-small cell lung cancer: a real-world study. Cancer Manage Res 12:3409–3417
Acknowledgements
We thank all the patients who involved in this trial, and this work was supported by the “Star of SJTU” plan Medical-Engineering cross fund of Shanghai Jiao Tong University (YG2019QNA48), the Western Medicine Guide Project of the Shanghai Committee of Science and Technology (18411968500) and Medical innovation project of Scientific and Technological innovation action plan of the Shanghai Committee of Science and Technology (21Y11913500).
Funding
This work was supported by the “Star of SJTU” plan Medical-Engineering cross fund of Shanghai Jiao Tong University (YG2019QNA48), the Western Medicine Guide Project of the Shanghai Committee of Science and Technology (18411968500) and Medical innovation project of Scientific and Technological innovation action plan of the Shanghai Committee of Science and Technology (21Y11913500).
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to the conception and design of the study. YCS, JL and FH contributed to data extraction, quality assessment, statistical analysis, and writing the report. JLQ, XYZ, RBZ and HZ contributed to data extraction and quality assessment. YCS, JL, FH, TQC and BHH contributed to interpretation of data and revision of the report. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethics approval
The clinical trial was approved by the independent ethics committee of each trial center and was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice requirements.
Consent to participate
All patients provided written informed consent in this study.
Consent to publish
NA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplement Figure 1
Progression-Free (A) and Overall Survival (B) of different histology type tumors in LM patients Supplementary file1 (TIFF 419 KB)
Supplement Figure 2
Overall Survival of different peripheral blood AST level (A) and ECOG PS score (B) in LM patients Supplementary file2 (TIFF 388 KB)
Rights and permissions
About this article
Cite this article
Shen, Y., Lu, J., Hu, F. et al. Effect and outcomes analysis of anlotinib in non-small cell lung cancer patients with liver metastasis: results from the ALTER 0303 phase 3 randomized clinical trial. J Cancer Res Clin Oncol 149, 1417–1424 (2023). https://doi.org/10.1007/s00432-022-03964-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03964-9