Abstract
Purpose
The dynamics of serum alpha-fetoprotein (AFP) level have been found to be a useful predictor of therapeutic responsiveness in patients with hepatocellular carcinoma (HCC). We evaluated whether AFP changes were able to accurately reflect imaging-based responses and predict prognosis in patients receiving therapies including immune-checkpoint inhibitors (ICIs).
Methods
A total of 108 HCC patients with baseline serum AFP ≥ 20 ng/mL who received ICI-based treatment were included. We evaluated AFP-based responses, coupled with radiographic responses by RECIST, at 6–10 (time-point 1, TP1) and 14–18 weeks (time-point 2, TP2) of therapy in terms of the change of AFP from baseline, with a > 20% decrease or increase in level corresponding to the AFP response and progression, respectively. We examined the correlations between AFP and imaging-based responses, and the prognostic implications of the AFP-based measure.
Results
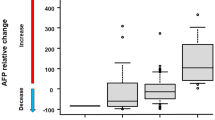
Based on AFP change, there were 24 and 20 responders and 74 and 24 progressors at TP1 and TP2, respectively. The AFP responders yielded radiological objective responses in 90.9% (10/11) and 93.8% (15/16) of the cases at TP1 and TP2, respectively, compared with only 1.4% and none, respectively, of the AFP progressors at the corresponding times. The agreement between progression by RECIST and increased AFP level at the two time-points was 93.8% and 95.0%, respectively. The accuracy of the AFP-based criterion for predicting radiologic response/progression was comparable at TP1 and TP2. Both “AFP responder” and “AFP progressor” at TP1 or TP2 independently predicted the overall survival of patients (adjusted hazard ratios [95% confidence intervals], 0.360 [0.174–0.743] and 0.315 [0.117–0.850]; and 2.525 [1.362–4.679] and 3.908 [1.563–9.769], respectively).
Conclusion
Our study suggests that on-treatment AFP changes can complement imaging findings and provide prognostic information for evaluating patients with AFP-producing HCC treated with ICI-based regimens.



Similar content being viewed by others
References
Borcoman E, Kanjanapan Y, Champiat S, Kato S, Servois V, Kurzrock R et al (2019) Novel patterns of response under immunotherapy. Ann Oncol 30(3):385–396
Brenner DJ, Eric J, Hall DP (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S et al (2017) Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23(8):1920–1928
Chan SL (2020) Hyperprogression in hepatocellular carcinoma: illusion or reality? J Hepatol 74:269–271
Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM et al (2009) New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol 27(3):446–452
Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M (2020) Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol 72(2):307–319
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382(20):1894–1905
Fujiwara N, Tateishi R, Akahane M, Taguri M, Minami T, Mikami S et al (2013) Changes in risk of immediate adverse reactions to iodinated contrast media by repeated administrations in patients with hepatocellular carcinoma. PLoS ONE 8(10):e76018
Greten TF, Lai CW, Li G, Staveley-O’Carroll KF (2019) Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology 156(2):510–524
Guite KM, Hinshaw JL, Ranallo FN, Lindstrom MJ, Lee FT Jr (2011) Ionizing radiation in abdominal CT: unindicated multiphase scans are an important source of medically unnecessary exposure. J Am Coll Radiol 8(11):756–761
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR et al (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67(1):358–380
Kang H, Lee HY, Lee KS, Kim JH (2012) Imaging-based tumor treatment response evaluation: review of conventional, new, and emerging concepts. Korean J Radiol 13(4):371–390
Kelley RK, Meyer T, Rimassa L, Merle P, Park JW, Yau T et al (2020) Serum alpha-fetoprotein levels and clinical outcomes in the phase III CELESTIAL study of cabozantinib versus placebo in patients with advanced hepatocellular carcinoma. Clin Cancer Res 26(18):4795–4804
Kim CG, Kim C, Yoon SE, Kim KH, Choi SJ, Kang B et al (2021) Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J Hepatol 74(2):350–359
Korean Liver Cancer Association-National Cancer Center (2019) Korean Liver Cancer Association-National Cancer Center Korea Practice guidelines for the management of hepatocellular carcinoma. Gut Liver 13(3):227–299
Koulakian H, Allaham W, Vilgrain V, Ronot M (2019) Non-measurable infiltrative HCC: is post-contrast attenuation on CT a sign of tumor response? Eur Radiol 29(8):4389–4399
Lee PC, Chao Y, Chen MH, Lan KH, Lee CJ, Lee IC et al (2020) Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers (basel) 12(1):182
Lee MS, Ryoo B-Y, Hsu C-H, Numata K, Stein S, Verret W et al (2020) Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol 21(6):808–820
Lee KH, Lee S, Park JH, Lee SS, Kim HY, Lee WJ et al (2021) Risk of hematologic malignant neoplasms from abdominopelvic computed tomographic radiation in patients who underwent appendectomy. JAMA Surg 156:343–351
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
Nishino M (2018) Tumor response assessment for precision cancer therapy: response evaluation on criteria in solid tumors and beyond. ASCO 38:1019–1029
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J et al (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11(4):317–370
Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F et al (2012) Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol 57(1):101–107
Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J et al (2009) Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol 27(34):5734–5742
Rimola J, Da Fonseca LG, Sapena V, Perello C, Guerrero A, Simo MT et al (2020) Radiological response to nivolumab in patients with hepatocellular carcinoma: a multicenter analysis of real-life practice. Eur J Radiol 135:109484
Robert C (2020) A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 11(1):3801
Rustin GJ, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T et al (2011) Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer 21(2):419–423
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26(7):1148–1159
Schoenfeld AJ, Hellmann MD (2020) Acquired resistance to immune checkpoint inhibitors. Cancer Cell 37(4):443–455
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18(3):e143–e152
Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL (2010) Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer 116(19):4590–4596
Shao YY, Liu TH, Hsu C, Lu LC, Shen YC, Lin ZZ et al (2019) Early alpha-foetoprotein response associated with treatment efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma. Liver Int 39(11):2184–2189
Sosna J (2019) Is RECIST version 1.1 reliable for tumor response assessment in metastatic cancer? Radiology 290(2):357–358
Suzuki C, Jacobsson H, Torkzad MR, Eriksson-Alm Y, Berg E, Kubo A et al (2008) Radiologic measurements of tumor response to treatment: practical approaches and limitations. RSNA 28:329–344
Tang J, Pearce L, O’Donnell-Tormey J, Hubbard-Lucey VM (2018) Trends in the global immuno-oncology landscape. Nat Rev Drug Discov 17(11):783–784
Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX (2009) Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist 14(7):717–725
Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM et al (2019) Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol 71(3):543–552
Funding
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (NRF-2017R1E1A1A01074298).
Author information
Authors and Affiliations
Contributions
HIK and JHS contributed to the study concept and design, the acquisition, analysis and interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. JL contributed to the acquisition of data and critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB No. 2019-1573) of the Asan Medical Center, which waived the requirement for informed consent.
Patients consent for publication
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, H.I., Lim, J. & Shim, J.H. Role of the alpha-fetoprotein response in immune checkpoint inhibitor-based treatment of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 148, 2069–2077 (2022). https://doi.org/10.1007/s00432-021-03727-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03727-y