Abstract
Objective
We aimed to review of literature on the clinical presentation, management and outcomes of pituitary apoplexy following gonadotrophic release hormone (GnRH) agonist administration for the treatment of prostate cancer.
Methods
We used PRISMA guidelines for our systematic review and included all English language original articles on pituitary apoplexy following GnRH agonist administration among prostate cancer patients from Jan 1, 1995 to Dec 31, 2020. Data on patient demographics, prostate cancer type, Gleason score at diagnosis, history of pituitary adenoma, clinical presentation, GnRH agonist, interval to pituitary apoplexy, laboratory evaluation at admission, radiologic findings, treatment of pituitary apoplexy, time to surgery if performed, pathology findings, and clinical/hormonal outcomes were collected and analyzed.
Results
Twenty-one patients with pituitary apoplexy met our inclusion criteria. The mean age of patients was 70 (60–83) years. Leuprolide was the most common used GnRH agonist, used in 61.9% of patients. Median duration to symptom onset was 5 h (few minutes to 6 months). Headache was reported by all patients followed by ophthalmoplegia (85.7%) and nausea/vomiting (71.4%). Three patients had blindness at presentation. Only 8 cases reported complete anterior pituitary hormone evaluation on presentation and the most common endocrine abnormality was FSH elevation. Tumor size was described only in 15 cases and the mean tumor size was 26.26 mm (18–48 mm). Suprasellar extension was the most common imaging finding seen in 7 patients. 71.4% of patients underwent pituitary surgery, while 23.8% were managed conservatively. Interval between symptoms onset to pituitary surgery was 7 days (1–90 days). Gonadotroph adenoma was most common histopathologic finding. Clinical resolution was comparable, while endocrine outcomes were variable among patients with conservative vs surgical management.
Conclusion
Although the use of GnRH agonists is relatively safe, it can rarely lead to pituitary apoplexy especially in patients with pre-existing pituitary adenoma. Physicians should be aware of this complication as it can be life threatening. A multidisciplinary team approach is recommended in treating individuals with pituitary apoplexy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary apoplexy (PA) is a rare but potentially life-threatening condition of bleeding into the pituitary gland that occurs in 0.6–10% of patients with known pituitary tumors (Nielsen et al. 2006). Only 10–40% of the cases of PA have an identified precipitating factor including the possibilities of angiographic procedures, orthopedic/cardiac surgeries, dynamic testing or medications such as GnRH agonists, anticoagulation therapy and dopamine agonists. Symptoms are broad and range from sudden onset of headache, ocular paresis, reduction in visual fields, and vomiting to altered mental status caused by the rapid enlargement of the pituitary gland due to bleeding and/or infarction usually within a tumor (Nielsen et al. 2006; Fraioli et al. 1990; Bonicki et al. 1993). Gonadotropin-releasing hormone (GnRH) agonists have been used in the management of different conditions including prostate cancer, precocious puberty, uterine bleeding due to endometriosis, as a transgender medicine, and fibroid treatment among others. Androgen deprivation therapy (ADT), consisting of GnRH agonists (leuprolide acetate, goserelin and triptorelin) or GnRH antagonists (degarelix and relugolix), is the treatment foundational treatment for advanced prostate cancer. GnRH agonists work by suppressing luteinizing hormone (LH) production and, therefore, the synthesis of testicular androgens. There have been few reported cases of PA following use of GnRH agonists in patients with prostate cancer. The diagnosis of PA requires a very high index of suspicion. The treatment of PA has remained controversial and was previously considered a neurosurgical emergency (Fraioli et al. 1990; Bonicki et al. 1993) but recent literature suggests the existence of spontaneous clinical recovery, and favors a conservative approach in individualized cases (Rajasekaran et al. 2011; Capatina et al. 2015). The outcomes of pituitary apoplexy are highly variable and can result in a rapid decline in clinical status ranging from subarachnoid hemorrhage or cerebral ischemia, or the patient may have a spontaneous recovery with or without any sequelae. Our study aimed to review the literature of pituitary apoplexy following androgen deprivation therapy for prostate cancer using GnRH agonists and characterize the clinical presentation, management and outcomes. Healthcare professionals should be aware of the association between GnRH agonist use and PA to enable an early recognition and prompt treatment.
Methods
Search strategy
We identified all the peer-reviewed published literature pertaining to pituitary apoplexy following GnRH agonist administration for the treatment of prostate cancer from Jan 1, 1995 to Dec 31, 2020. We adopted Prisma guidelines for our systematic review and used electronic database of MEDLINE, Web of Science, Scopus, and Cochrane Library to identify all the related articles published by Dec 31, 2021. The following keywords were used: “Pituitary Apoplexy”, “Cerebral Hemorrhage”, “Pituitary Diseases”, “Pituitary Neoplasms”, gonadotropin-releasing hormone (GnRH), “Gonadotropin-Releasing Hormone”, “gonadotropin-releasing hormone agonist”, “Prostate Cancer” and “Prostatic Neoplasms”. The identified articles were assessed for inclusion independently by two authors (GE, RR) and divergences were unified through discussion. Eligibility criterion for inclusion in the review was the event of pituitary apoplexy following GnRH agonist administration for the treatment of prostate cancer. Only manuscripts in English language were included in the study. A Prisma flow chart showing the process of identification of articles via databases is shown in Fig. 1.
Data collection
The information extracted from the selected publications were as follows: author, year, study design, patient demographics, prior history of pituitary adenoma, clinical signs/symptoms at presentation, type of GnRH agonist used, interval to pituitary apoplexy following GnRH agonist use, laboratory evaluation on admission, radiologic findings on presentation, treatment method, time to surgery after presentation, pathology findings, and clinical/hormonal outcome.
Data analysis
Categorical variables were reported as percentage and continuous variables as means or medians. For the parametric variables, we used t test for analysis. For the non-parametric variable, we used Wilcoxon test for analysis. All the statistical analyses were performed using SPSS 26.0. Clinical features and efficacy outcomes were not statistically analyzed further due to small sample size and inconsistency in data reporting.
Results
Baseline characteristics (Tables 1 and 2)
We identified a total of 66 published articles between 1995 and 2020 on database search and 21 cases fulfilled inclusion criteria (Ando et al. 1995; Chanson and Schaison 1995; Morsi et al. 1996; Reznik et al. 1997; Eaton et al. 2001; Spengos et al. 2002; Blaut et al. 2006; Davis et al. 2006; Massoud et al. 2006; Hands et al. 2007; Guerra et al. 2010; Sinnadurai et al. 2010; Ito 2011; Huang et al. 2013; Babbo et al. 2014; Guerrero-Pérez et al. 2015; Sasagawa et al. 2015; Fabiano and George 2016; Keane et al. 2016; Tanios et al. 2017; Barbosa et al. 2020). The mean age of the patients was 70 years. Among the reports which disclosed details about prostate cancer, 38.1% were local or locally advanced prostate cancer and 14.3% were recurrence after prior local treatments. Only 5 cases reported Gleason score and PSA levels at presentation were inconsistently reported. Only 3 patients (14.3%) had a prior history of pituitary adenoma.
Sixty-two (62) % of the patients received leuprolide. The doses of leuprolide associated with pituitary apoplexy varied; three patients used 11.25 mg dose, two were on 3.75 mg, two received 45 mg and one each on 2.5, 22.5, and 30 mg; in three patients the dose of leuprolide was not reported. Among the five patients who received goserelin, four had 3.6 mg subcutaneous dose, while one patient had a subcutaneous implant. One patient each received triptorelin 3.75 and 22.5 mg triptorelin, while in one patient dose was not reported. 7 out of the 21 patients also received prior/concurrent antiandrogen therapy.
Clinical presentation (Table 3)
Thirteen (61.9%) patients developed symptoms within 24 h of administration of GnRH agonist, while eight patients (38.09%) developed symptoms between 1 day and 6 months. Median duration to onset of symptoms or diagnosis of apoplexy post-GnRH administration was 5 h. Four patients who were on prior/concurrent anti-androgen therapy had early onset PA.
All patients had headache as the initial presenting symptom. Twenty patients (95.2%) had visual symptoms at presentation with ophthalmoplegia present among 18 and visual field defect in 9 patients. Three patients had blindness at presentation.
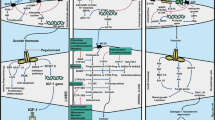
Laboratory findings at presentation (Table 3, Fig. 2)
Only 8 patients had reported complete anterior pituitary hormone evaluation and 16 cases had at least one pituitary hormone evaluation reported. The most common endocrine abnormality on presentation was follicle-stimulating hormone (FSH) elevation (n = 6) followed by thyroid-stimulating hormone (TSH) deficiency (n = 6) and low prolactin levels (n = 5). One patient had hyponatremia secondary to Syndrome of Inappropriate Anti Diuretic Hormone (SIADH) and another patient had central diabetes insipidus.
Imaging findings (Table 3)
Tumor size was described in only 15 cases. The mean maximum dimension was 26.26 mm (range: 18–48 mm). Nine cases described the tumor volume, with a mean volume of 9600 mm3 (range: 4000–50,400 mm3). Seven patients had suprasellar extension, 5 with cavernous sinus extension and 4 had both suprasellar and cavernous sinus extension. Infrasellar extension was rare with only one reported case.
Management
Among 21 patients, 15 (71.4%) underwent pituitary surgery, while 5 (23.8%) were conservatively managed. One patient had surgery planned but not reported as completed. Among patients managed with pituitary surgery, the interval between symptoms onset to pituitary surgery was approximately 7 days (range: 1–90 days).
Pathology
Pathologic findings were described among 12 out of the 15 patients who underwent surgical treatment for pituitary apoplexy. 10 tissue samples were LH and FSH positivity by immunohistochemistry, while one was GH positive. Two tissue samples were inconclusive on analysis with hematoma and necrotic tissue identified.
Outcomes (Tables 4 and 5)
All patient who underwent pituitary surgery had complete or partial resolution of symptoms. Conservative management resulted in complete resolution of symptoms in 3 patients, while 1 patient had a persistent visual field defect. Endocrine outcomes were variable for surgical as well as conservative management. Patients with hormonal abnormalities at presentation continued to have persistent abnormalities post-management, whether surgical or conservative. Two patients who underwent surgical management died. One patient died of cardiac arrest 12-day post-surgery and another patient died of complications from malignant melanoma 8 months later (Morsi et al. 1996; Eaton et al. 2001).
Post-PA prostate cancer treatment
Treatment of prostate cancer after PA was not described in 11 patients. Among the five patients treated conservatively, one patient received radiation treatment for prostate cancer following PA and the management of prostate cancer was not described in the rest (Barbosa et al. 2020).
Among 15 patients treated with surgery, 7 did not describe further management of prostate cancer. One patient with locally advanced prostate cancer had a normal testosterone level after surgery and was on observation for prostate cancer with stable prostate specific antigen (PSA) levels after 1 year (Massoud et al. 2006). GnRH agonist rechallenge was attempted in 4 patients. As reported by Reznik et al., leuprolide rechallenge failed to suppress LH/FSH and testosterone. This patient was treated with flutamide and underwent orchiectomy (Reznik et al. 1997). The other 3 patients had successful rechallenge with GnRH agonists (Blaut et al. 2006; Davis et al. 2006; Sasagawa et al. 2015).
Two patients received androgen receptor blocker—bicalutamide only (Sinnadurai et al. 2010; Ito 2011), while one patient had radiation treatment in addition to bicalutamide (Babbo et al. 2014). Degarelix and radiation was used for prostate cancer management in the report from Keane et al. (2016).
Discussion
Our review focused on patients receiving a GnRH agonist as part of ADT for treatment of prostate cancer who developed PA. The mean age of patients included in the series corresponds to the peak age of prostate cancer diagnosis, which is between 65 and 74 years (National Cancer Institute’s Surveillance, Epidemiology, and End Results program 13). PA was reported with 3 of the currently FDA approved GnRH agonists—goserelin, leuprolide and triptorelin—and there were no available reports of associated PA with buserelin or histrelin.
A history of pituitary adenoma prior to presentation was reported only in 3 cases. However, previously undiagnosed adenomas could have been present in additional cases considering the estimated prevalence of pituitary adenoma to be 77.6 per 100,000 in general population (Fernandez et al. 2010). Although early presentation (within 24 h) was more common, unusually late presentation at 8 weeks and 6 months was also observed (Spengos et al. 2002; Sinnadurai et al. 2010). Clinical presentation was variable. Headache was the most frequent and the earliest of symptoms, which occurred in all of the patients described. This symptom could be explained by the stretching and stimulation of the hypophyseal capsule, with increased intracranial pressure and/or hemorrhage into the subarachnoid space (Li et al. 2020). Cranial nerve palsies and systemic symptoms such as nausea and vomiting were also common.
Though limited in number of case reports have suggested a causative role of GnRH agonists in development of PA, but the exact pathophysiology is not well established. When administered, GnRH agonists binds to GnRH receptors on pituitary gonadotropin-secreting cells and stimulate gonadotropin secretion: FSH and LH. The GnRH agonists are more potent and have a longer half-life than native GnRH. With the initiation of GnRH agonist treatment there is a dramatic and transient surge of LH and FSH before the levels fall. This LH flare, might, in addition, correlate to transient prostate cancer growth as well as with an increase in size of occult pituitary tumors (Rajasekaran et al. 2011; Capatina et al. 2015). The unique rich vasculature of the pituitary gland, combined with the fragility of the tumor blood vessels can further exacerbate the hemorrhagic tendency. The expanding pituitary tumor, which results from the hemorrhage in the adenoma leads to the compression of the infundibular and hypophyseal vessels, which can ultimately results in pituitary infarction (Blaut et al. 2006; Li et al. 2020; Abbott and Kirkby 2004; Briet et al. 2015; Powell et al. 1974). Another study suggested that pharmacological agents may stimulate the growth of the pituitary adenoma and also can have a direct impact on a tumor’s microvasculature leading to vasoconstriction with subsequent impairment of oxygen and nutritional supply of pituitary adenomas (Okuda et al. 1994). Gonadotroph adenomas, the majority of which are non-functioning adenomas, are the most common adenomas associated with the occurrence of PA. This is consistent with our review, where the majority of PA were found to be gonadotroph adenomas on histopathological examination and were silent pituitary adenoma until presentation as PA.
Endocrine hormone evaluation at the onset of PA was mentioned in most cases, however, was incomplete especially regarding values of ACTH and GH/IGF1. Many of the cases also had missing information on pre- and post-treatment for prolactin, TSH, and FSH/LH. Given the patient’s age group, we would expect some degree of elevation in the gonadotropin hormones. Despite this, FSH elevation was present in only 6 cases. Gonadotropin adenoma was also the most common finding on pathology (silent or non-silent). Although the majority of patient’s underwent pituitary surgery, the factors determining choice of surgical vs conservative management were unclear. Irrespective of management choice, most patients had partial or complete resolution of symptoms. The development of pituitary hormone deficiency is an expected outcome of surgical management, but was also present in the conservatively managed patients.
Author’s recommendations
Prostate cancer will rarely metastasize to the brain and brain imaging is not routinely done for these patients. A screening pituitary MRI before the use of GnRH agonist is not cost effective, but we suggest a focused review of the pituitary gland from prior radiological images if available. In Babbo et al.’s report, a patient with prior pituitary microadenoma who underwent ADT did not develop PA which suggests that not all patients with pituitary adenoma will develop PA with ADT (Babbo et al. 2014). Hence, the presence of a pituitary adenoma should not be considered as an absolute contraindication for ADT. In these patients as well as those who are at higher risk of PA with GnRH agonist use, GnRH antagonists such as degarelix and relugolix may be considered. The role of concurrent use of antiandrogen therapies such as bicalutamide, flutamide, or cyproterone acetate in this scenario is not clear. As bicalutamide and flutamide only have peripheral activity due to poor penetration of blood brain barrier, their use may have minimal to no effect on pituitary gland (Furr 1996; Sardanons 1989). On the other hand, cyproterone acetate (not currently FDA approved in United States for use in patients with prostate cancer) is known to cross blood–brain barrier (Denmeade and Isaacs 2002). Cyproterone acetate is also known to worsen prolactinoma and hence has the potential to result in PA among patients with occult or known pituitary adenoma (Raj et al. 2018). In our review, 3 patients with prior/concurrent use of cyproterone acetate developed PA.
Patients who develop visual symptoms, headache and nausea or vomiting after initiation of ADT should promptly undergo a pituitary hormonal workup and brain imaging studies for the diagnosis and early appropriate management of PA. The course of PA is variable and management should be individualized. A thorough clinical evaluation by a multidisciplinary team of specialists including neurosurgeons, endocrinologists, neurologist and oncologist is required, especially in patients with evidence of pituitary macroadenomas prior to the start of these agents. The aim of the therapy is to improve the patient’s symptoms and relieve the compression of the surrounding structures. This can be achieved rapidly by surgical intervention while being mindful of the risks of surgical complications. Other options include conservative management with high dose steroids, especially in patients with corticotropic deficiency which is frequent (Briet et al. 2015). A discussion of risks and benefits with the patient is recommended prior to choosing the management route. The available case reports do not mention treatment-related complications or long-term outcomes of these patients. We have summarized key elements in diagnosis and management of PA in patients treated with GnRH agonists in Table 6.
There are limitations for this retrospective review of the literature. The case reports lack uniformity and completeness in describing details of laboratory evaluation and management. The language was restricted to English leading to the exclusion of few case reports. In addition, with very few reported cases in the literature and short follow-up, pursuing a full outcome analysis is not possible.
Conclusion
Use of GnRH agonist in patients with prostate cancer can lead to endocrine complications including PA. Physicians should be aware of this complication as it is considered life threatening and carefully observe the patients during administration of these pharmacological agents. In addition, they should inform the patients regarding warning signs and symptoms that may occur with time, especially in the setting of a prior pituitary adenoma. A careful evaluation by a multidisciplinary team including endocrinologist, oncologist and neurosurgeons is needed if PA occurs; an understanding of the risk factors can aid in an early diagnosis and the management of these patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon request. The authors declare that all data supporting the findings of this study are available within the paper.
Abbreviations
- n:
-
Number
- C:
-
Caucasian
- A:
-
Asian
- AA:
-
African–American
- ND:
-
Not described
- H:
-
Headache
- N/V:
-
Nausea/vomiting
- OP:
-
Ophthalmoplegia
- VF:
-
Visual field defect
- VA:
-
Visual acuity defect
- P:
-
Photophobia
- F:
-
Fever
- AMS:
-
Altered mental status
- EP:
-
Eye pain
- B:
-
Blindness
- M:
-
Meningism
- ACTH:
-
Adrenocorticotrophic hormone
- TSH:
-
Thyroid stimulating hormone
- LH:
-
Luteinizing hormone
- FSH:
-
Follicle secreting hormone
- GH:
-
Growth hormone
- IGF-1:
-
Insulin-like growth factor-1
- CR:
-
Complete resolution
- PR:
-
Partial resolution
- NR:
-
No resolution
References
Abbott J, Kirkby GR (2004) Acute visual loss and pituitary apoplexy after surgery. BMJ 329(7459):218–219
Ando S, Hoshino T, Mihara S (1995) Pituitary apoplexy after goserelin. Lancet 345(8947):458
Babbo A, Kalapurakal GT, Liu B, Bajramovic S, Chandler JP, Garnett J et al (2014) The presence of a pituitary tumor in patients with prostate cancer is not a contraindication for leuprolide therapy. Int Urol Nephrol 46(9):1775–1778
Barbosa M, Paredes S, Machado MJ, Almeida R, Marques O (2020) Pituitary apoplexy induced by gonadotropin-releasing hormone agonist administration: a rare complication of prostate cancer treatment. Endocrinol Diabetes Metab Case Rep 2020:20
Blaut K, Wisniewski P, Syrenicz A, Sworczak K (2006) Apoplexy of clinically silent pituitary adenoma during prostate cancer treatment with LHRH analog. Neuro Endocrinol Lett 27(5):569–572
Bonicki W, Kasperlik-Zaluska A, Koszewski W, Zgliczynski W, Wislawski J (1993) Pituitary apoplexy: endocrine, surgical and oncological emergency. Incidence, clinical course and treatment with reference to 799 cases of pituitary adenomas. Acta Neurochir (wien) 120(3–4):118–122
Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P (2015) Pituitary apoplexy. Endocr Rev 36(6):622–645
Capatina C, Inder W, Karavitaki N, Wass JA (2015) Management of endocrine disease: pituitary tumour apoplexy. Eur J Endocrinol 172(5):R179–R190
Chanson P, Schaison G (1995) Pituitary apoplexy caused by GnRH-agonist treatment revealing gonadotroph adenoma. J Clin Endocrinol Metab 80(7):2267–2268
Davis A, Goel S, Picolos M, Wang M, Lavis V (2006) Pituitary apoplexy after leuprolide. Pituitary 9(3):263–265
Denmeade SR, Isaacs JT (2002) A history of prostate cancer treatment. Nat Rev Cancer 2(5):389–396
Eaton HJ, Phillips PJ, Hanieh A, Cooper J, Bolt J, Torpy DJ (2001) Rapid onset of pituitary apoplexy after goserelin implant for prostate cancer: need for heightened awareness. Intern Med J 31(5):313–314
Fabiano AJ, George S (2016) Pituitary apoplexy after initial Leuprolide injection. World Neurosurg 95(616):e7–e9
Fernandez A, Karavitaki N, Wass JA (2010) Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (oxf) 72(3):377–382
Fraioli B, Esposito V, Palma L, Cantore G (1990) Hemorrhagic pituitary adenomas: clinicopathological features and surgical treatment. Neurosurgery 27(5):741–747 (discussion 7-8)
Furr BJ (1996) The development of Casodex (bicalutamide): preclinical studies. Eur Urol 29(Suppl 2):83–95
Guerra Y, Lacuesta E, Marquez F, Raksin PB, Utset M, Fogelfeld L (2010) Apoplexy in non functioning pituitary adenoma after one dose of leuprolide as treatment for prostate cancer. Pituitary 13(1):54–59
Guerrero-Pérez F, Marengo AP, Planas-Vilaseca A, Flores-Escobar V, Villabona-Artero C (2015) Pituitary apoplexy induced by triptorelin in patient with prostate cancer. Endocrinol Nutr 62(8):411–412
Hands KE, Alvarez A, Bruder JM (2007) Gonadotropin-releasing hormone agonist-induced pituitary apoplexy in treatment of prostate cancer: case report and review of literature. Endocr Pract 13(6):642–646
Huang TY, Lin JP, Lieu AS, Chen YT, Chen HS, Jang MY et al (2013) Pituitary apoplexy induced by Gonadotropin-releasing hormone agonists for treating prostate cancer-report of first Asian case. World J Surg Oncol 11:254
Ito Y (2011) Unexpected enlargement of clinically silent pituitary gonadotroph adenoma induced by goserelin acetate given as treatment for prostate cancer. Int J Urol 18(1):83–84
Keane F, Egan AM, Navin P, Brett F, Dennedy MC (2016) Gonadotropin-releasing hormone agonist-induced pituitary apoplexy. Endocrinol Diabetes Metab Case Rep 2016:160021
Li Y, Qian Y, Qiao Y, Chen X, Xu J, Zhang C et al (2020) Risk factors for the incidence of apoplexy in pituitary adenoma: a single-center study from southwestern China. Chin Neurosurg J 6:20
Massoud W, Paparel P, Lopez JG, Perrin P, Daumont M, Ruffion A (2006) Discovery of a pituitary adenoma following treatment with a gonadotropin-releasing hormone agonist in a patient with prostate cancer. Int J Urol 13(1):87–88
Morsi A, Jamal S, Silverberg JD (1996) Pituitary apoplexy after leuprolide administration for carcinoma of the prostate. Clin Endocrinol (oxf) 44(1):121–124
Nielsen EH, Lindholm J, Bjerre P, Christiansen JS, Hagen C, Juul S et al (2006) Frequent occurrence of pituitary apoplexy in patients with non-functioning pituitary adenoma. Clin Endocrinol (oxf) 64(3):319–322
Okuda O, Umezawa H, Miyaoka M (1994) Pituitary apoplexy caused by endocrine stimulation tests: a case report. Surg Neurol 42(1):19–22
Powell DF, Baker HL Jr, Laws ER Jr (1974) The primary angiographic findings in pituitary adenomas. Radiology 110(3):589–595
Raj R, Korja M, Koroknay-Pal P, Niemela M (2018) Multiple meningiomas in two male-to-female transsexual patients with hormone replacement therapy: a report of two cases and a brief literature review. Surg Neurol Int 9:109
Rajasekaran S, Vanderpump M, Baldeweg S, Drake W, Reddy N, Lanyon M et al (2011) UK guidelines for the management of pituitary apoplexy. Clin Endocrinol (oxf) 74(1):9–20
Reznik Y, Chapon F, Lahlou N, Deboucher N, Mahoudeau J (1997) Pituitary apoplexy of a gonadotroph adenoma following gonadotrophin releasing hormone agonist therapy for prostatic cancer. J Endocrinol Invest 20(9):566–568
Sardanons ML, de LasHeras MA, Calandra RS, Solano AR, Podesta EJ (1989) Effect of the antiandrogen flutamide on pituitary LH content and release. Neuroendocrinology 50(2):211–216
Sasagawa Y, Tachibana O, Nakagawa A, Koya D, Iizuka H (2015) Pituitary apoplexy following gonadotropin-releasing hormone agonist administration with gonadotropin-secreting pituitary adenoma. J Clin Neurosci 22(3):601–603
Sinnadurai M, Cherukuri RK, Moses RG, Nasser E (2010) Delayed pituitary apoplexy in patient with advanced prostate cancer treated with gonadotrophin-releasing hormone agonists. J Clin Neurosci 17(9):1201–1203
Spengos K, Pavlopoulos C, Grivas A (2002) Pituitary haemorrhage after leuprolide therapy for prostatic cancer, clinically imitating acute subarachnoidal haemorrhage. Cerebrovasc Dis 14(3–4):272
Tanios G, Mungo NA, Kapila A, Bajaj K (2017) Pituitary apoplexy: a rare complication of leuprolide therapy in prostate cancer treatment. BMJ Case Rep 2017:bcr2016218514
Acknowledgements
Our research was supported by the Research Communication Office Markey Shared Resource Facility, NCI Cancer Center Support Grant (P30 CA177558).
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector but Open access.
Author information
Authors and Affiliations
Contributions
RR, GE, AA, DU, AJ were directly involved in conceptualization, data collection and data analysis of the study. All the authors contributed in writing the manuscript. RC and ZM supervised and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raj, R., Elshimy, G., Jacob, A. et al. Pituitary apoplexy induced by gonadotropin-releasing hormone (GnRH) agonist administration for treatment of prostate cancer: a systematic review. J Cancer Res Clin Oncol 147, 2337–2347 (2021). https://doi.org/10.1007/s00432-021-03697-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03697-1