Abstract
Purpose
The purpose of our study was to compare genomic changes in sinonasal intestinal-type adenocarcinoma (sITAC) and colorectal adenocarcinoma (CRC), as they are histomorphologically indistinguishable. This can cause diagnostic difficulties as sinonasal tumours initially diagnosed as sITAC may represent metastasis from CRC, a frequent cancer. Previous studies have not uncovered the underlying mechanism behind the histomorphological resemblance.
Methods/patients
Tissue samples from all consecutive patients with sITAC at our facility (20 patients) were compared to samples from 20 patients with CRC as well as samples from 2 patients with both CRC and sinonasal tumours. DNA sequencing was performed using Illumina TruSight Oncology 500 panel consisting of 523 cancer-associated genes. Frequent mutations were inspected manually using the Integrative Genomics Viewer.
Results
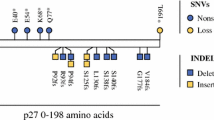
Several well-known cancer-associated genes were mutated in the CRC group, but also in the sinonasal ITAC group. These genes included APC mutated in 65% of the CRC group and 37% of the sinonasal ITAC group, and TP53 mutated in 65% of CRC samples and 58% of ITAC samples. These shared mutations may explain the histomorphological similarities. Successful DNA sequencing was performed on the colorectal sample from one of the two patients with both CRC and sinonasal tumour. Comparing mutations in these samples from one patient we have shown that the sinonasal tumour in all probability was a CRC metastasis.
Conclusion
We have identified several genetic similarities between sITAC and CRC. This discovery brings us closer to understanding mechanisms behind the development of sITAC—and hopefully in the future targeted therapy.




Similar content being viewed by others
Change history
08 December 2020
A Correction to this paper has been published: https://doi.org/10.1007/s00432-020-03473-7
References
Alonso-Sardón M, Chamorro A-J, Hernández-García I et al (2015) Association between occupational exposure to wood dust and cancer: a systematic review and meta-analysis. PLoS ONE 10(7):e0133024. https://doi.org/10.1371/journal.pone.0133024
Athar PPBSH, Norhan NBA, Bin SL, Rose IBM, Ramli RB (2008) Metastasis to the sinonasal tract from sigmoid colon adenocarcinoma. Ann Acad Med Singapore 37(9):788–790
Balch C, Ramapuram JB, Tiwari AK (2017) The epigenomics of embryonic pathway signaling in colorectal cancer. Front Pharmacol 8(MAY):1–7. https://doi.org/10.3389/fphar.2017.00267
Bandrés E, Malumbres R, Cubedo E et al (2007) A gene signature of 8 genes could identify the risk of recurrence and progression in Dukes’ B colon cancer patients. Oncol Rep 17(5):1089–1094. https://doi.org/10.3892/or.17.5.1089
Binazzi A, Ferrante P, Marinaccio A (2015) Occupational exposure and sinonasal cancer: a systematic review and meta-analysis. BMC Cancer 15(1):49. https://doi.org/10.1186/s12885-015-1042-2
Bonnet E, Moutet ML, Baulard C et al (2018) Performance comparison of three DNA extraction kits on human whole-exome data from formalin-fixed paraffin-embedded normal and tumor samples. PLoS ONE 13(4):1–20. https://doi.org/10.1371/journal.pone.0195471
Bornholdt J, Hansen J, Steiniche T et al (2008) K-rasmutations in sinonasal cancers in relation to wood dust exposure. BMC Cancer 8(1):53. https://doi.org/10.1186/1471-2407-8-53
Brenner H, Kloor M, Pox CP (2014) Colorectal cancer. Lancet 383:1490–1502. https://doi.org/10.1080/00325481.1992.11701292
Bussi M, Gervasio CF, Riontino E et al (2002) Study of ethmoidal mucosa in a population at occupational high risk of sinonasal adenocarcinoma. Acta Otolaryngol 122(2):197–201. https://doi.org/10.1080/00016480252814225
Cama E, Agostino S, Ricci R, Scarano E (2002) A rare case of metastases to the maxillary sinus from sigmoid colon adenocarcinoma. ORL J Otorhinolaryngol Relat Spec 64(5):364–367
Carrick DM, Mehaffey MG, Sachs MC et al (2015) Robustness of next generation sequencing on older formalin-fixed paraffin-embedded tissue. PLoS ONE 10(7):3–10. https://doi.org/10.1371/journal.pone.0127353
Chakrabarty S, Varghese VK, Sahu P et al (2017) Targeted sequencing-based analyses of candidate gene variants in ulcerative colitis-associated colorectal neoplasia. Br J Cancer 117(1):136–143. https://doi.org/10.1038/bjc.2017.148
Chang M-H, Kuo Y-J, Ho C-Y, Kuan EC, Lan M-Y (2019) Metastatic tumors of the sinonasal cavity: a 15-year review of 17 cases. J Clin Med 8(4):539. https://doi.org/10.3390/jcm8040539
Chen C, Méndez E, Houck J et al (2005) Adult height and head and neck cancer: a pooled analysis within the INHANCE Consortium. Hum Pathol 36(2):1391. https://doi.org/10.1002/HED
Conill C, Vargas M, Valduvieco I, Fernández PL, Cardesa A, Capurro S (2009) Metastasis to the nasal cavity from primary rectal adenocarcinoma. Clin Transl Oncol 11(2):117–119. https://doi.org/10.1007/s12094-009-0325-y
Donhuijsen K, Hannig H, Schroeder HG, Korinth D, Petersen I (2007) Synchronous adenocarcinomas of intestinal type of the inner nose and the colon. Hum Pathol 38(2):373–377. https://doi.org/10.1016/j.humpath.2006.08.004
Donhuijsen K, Kollecker I, Petersen P, Gassler N, Schulze J, Schroeder H-G (2016) Metastatic behaviour of sinonasal adenocarcinomas of the intestinal type (ITAC). Eur Arch Otorhinolaryngol 273(3):649–654. https://doi.org/10.1007/s00405-015-3596-7
Dow LE, O’Rourke KP, Simon J et al (2015) Reestablishes crypt homeostasis in colorectal cancer. Cell 161(7):1539–1552. https://doi.org/10.1016/j.cell.2015.05.033.Apc
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J et al (2015) Reprint of: cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 51(9):1201–1202. https://doi.org/10.1016/j.ejca.2015.05.004
Franchi A, Palomba A, Fondi C et al (2011) Immunohistochemical investigation of tumorigenic pathways in sinonasal intestinal-type adenocarcinoma. A tissue microarray analysis of 62 cases. Histopathology 59(1):98–105. https://doi.org/10.1111/j.1365-2559.2011.03887.x
Franchi A, Palomba A, Miligi L et al (2014) Intestinal metaplasia of the sinonasal mucosa adjacent to intestinal-type adenocarcinoma. A morphologic, immunohistochemical, and molecular study. Virchows Arch 466(2):161–168. https://doi.org/10.1007/s00428-014-1696-1
Franchi A, Palomba A, Miligi L et al (2015) Intestinal metaplasia of the sinonasal mucosa adjacent to intestinal-type adenocarcinoma. A morphologic, immunohistochemical, and molecular study. Virchows Arch 466(2):161–168. https://doi.org/10.1007/s00428-014-1696-1
Frattini M, Perrone F, Suardi S et al (2006) Phenotype-genotype correlation: challenge of intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Head Neck 28(10):909–915. https://doi.org/10.1002/hed.20433
García-Inclán C, López F, Pérez-Escuredo J et al (2012) EGFR status and KRAS/BRAF mutations in intestinal-type sinonasal adenocarcinomas. Cell Oncol 35(6):443–450. https://doi.org/10.1007/s13402-012-0103-7
Ghosh N, Hossain U, Mandal A, Sil PC (2019) The Wnt signaling pathway: a potential therapeutic target against cancer. Ann NY Acad Sci 1443(1):54–74. https://doi.org/10.1111/nyas.14027
Hadfield EH (1970) A study of adenocarcinoma of the paranasal sinuses in woodworkers in the furniture in woodworkers in the furniture industry. Ann R Coll Surg Engl 46:301–319
Hankey W, Frankel WL, Groden J (2018) Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Cancer Metastasis Rev 37(1):159–172. https://doi.org/10.1016/j.physbeh.2017.03.040
Hoeben A, van der Winkel L, Hoebers F et al (2016) Intestinal-type sinonasal adenocarcinomas: the road to molecular diagnosis and personalized treatment. Head Neck 36(10):1564–1570. https://doi.org/10.1002/HED
Hugen N, Van de Velde CJH, De Wilt JHW, Nagtegaal ID (2014) Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 25(3):651–657. https://doi.org/10.1093/annonc/mdt591
Hwang S, Chan D, Wang S, Tan K (2020) Nasal cavity metastasis from colorectal cancer represents end-stage disease and should be palliated. Ann Coloproctol 36(2):119–121. https://doi.org/10.3393/ac.2019.03.04 (Epub ahead of print)
Kariv R, Caspi M, Fliss-Isakov N et al (2020) Resorting the function of the colorectal cancer gatekeeper adenomatous polyposis coli. Int J Cancer 146(4):1064–1074. https://doi.org/10.1002/ijc.32557
Kim MS, Chung NG, Kang MR, Yoo NJ, Lee SH (2011) Genetic and expressional alterations of CHD genes in gastric and colorectal cancers. Histopathology 58(5):660–668. https://doi.org/10.1111/j.1365-2559.2011.03819.x
Leivo I (2017) Intestinal-type adenocarcinoma: classification, immunophenotype, molecular features and differential diagnosis. Head Neck Pathol 11(3):295–300. https://doi.org/10.1007/s12105-017-0800-7
Leoncini E, Ricciardi W, Cadoni G et al (2014) Adult height and head and neck cancer: a pooled analysis within the INHANCE Consortium. Head Neck 36(10):1391. https://doi.org/10.1002/HED
Lim EH, Mohamad I, Ramli RR, Krishna BVM (2017) Infratemporal fossa metastasis of colorectal carcinoma. Egypt J Ear Nose Throat Allied Sci 18(2):183–185. https://doi.org/10.1016/j.ejenta.2016.12.012
Liu JC, Ferreira CG, Yusufzai T (2015) Human CHD2 is a chromatin assembly ATPase regulated by its chromo- and DNA-binding domains. J Biol Chem 290(1):25–34. https://doi.org/10.1074/jbc.M114.609156
López F, García Inclán C, Pérez-Escuredo J et al (2012) KRAS and BRAF mutations in sinonasal cancer. Oral Oncol 48(8):692–697. https://doi.org/10.1016/j.oraloncology.2012.02.018
López F, Devaney KO, Hanna EY, Rinaldo A, Ferlito A (2016) Metastases to nasal cavity and paranasal sinuses. Head Neck 38:1847–1854. https://doi.org/10.1002/HED
Lund V, Stammberger H, Nicolai P, Castelnuovo P (2010) European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl 22:1–143
Mármol I, Sánchez-de-Diego C, Dieste AP, Cerrada E, Yoldi MJR (2017) Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. https://doi.org/10.3390/ijms18010197
Murnyák B, Hortobágyi T (2016) Immunohistochemical correlates of TP53 somatic mutations in cancer. Oncotarget 7(40):64910–64920. https://doi.org/10.18632/oncotarget.11912
Muzny DM, Bainbridge MN, Chang K et al (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487(7407):330–337. https://doi.org/10.1038/nature11252
Nagarajan P, Onami TM, Rajagopalan S, Kania S, Donnell R, Venkatachalam S (2009) Damage response signaling and tumorigenesis. Oncogene 28(8):1053–1062. https://doi.org/10.1038/onc.2008.440.Role
Niederreither K, Harbers M, Chambon P, Dolle P (1998) Expression of T: G mismatch-specific thymidine-DNA glycosylase and DNA methyl transferase genes during development and tumorigenesis. Oncogene 17(12):1577–1585. https://doi.org/10.1038/sj.onc.1202072
Ortiz-Rey JA, Alvarez C, San Miguel P, Iglesias B, Anton I (2005) Expression of CDX2, cytokeratins 7 and 20 in sinonasal intestinal-type adenocarcinoma. Appl Immunohistochem Mol Morphol AIMM 13(2):142–146
Perrone F, Oggionni M, Birindelli S et al (2003) TP53, P14ARF, P16INK4aand H-ras gene molecular analysis in intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Int J Cancer 105(2):196–203. https://doi.org/10.1002/ijc.11062
Pinato DJ, Krell J, Wasan H, Sharma R (2011) Neoplastic infiltration of the sphenoid wing: a rare manifestation of metastatic colorectal cancer. Asia Pac J Clin Oncol 7(4):399–400. https://doi.org/10.1111/j.1743-7563.2011.01434.x
Porru M, Pompili L, Caruso C, Biroccio A, Leonetti C (2018) Targeting kras in metastatic colorectal cancer: current strategies and emerging opportunities. J Exp Clin Cancer Res 37(1):1–10. https://doi.org/10.1186/s13046-018-0719-1
Projetti F, Durand K, Chaunavel A et al (2013) Epidermal growth factor receptor expression and KRAS and BRAF mutations: Study of 39 sinonasal intestinal-type adenocarcinomas. Hum Pathol 44(10):2116–2125. https://doi.org/10.1016/j.humpath.2013.03.019
Projetti F, Mesturoux L, Coulibaly B et al (2015) Study of MET protein levels and MET gene copy number in 72 sinonasal intestinal-type adenocarcinomas. Head Neck 36(10):1563–1568. https://doi.org/10.1002/HED
Rampias T, Karagiannis D, Avgeris M et al (2019) The lysine-specific methyltransferase KMT 2C/MLL 3 regulates DNA repair components in cancer. EMBO Rep 20(3):1–20. https://doi.org/10.15252/embr.201846821
Re M, Magliulo G, Tarchini P et al (2011) p53 and BCL-2 over-expression inversely correlates with histological differentiation in occupational ethmoidal intestinal-type sinonasal adenocarcinoma. Int J Immunopathol Pharmacol 24(3):603–609. https://doi.org/10.1177/039463201102400306
Robinson JT, Thorvaldsdóttir H, Winckler W et al (2011) Integrative genome viewer. Nat Biotechnol 29(1):24–26. https://doi.org/10.1038/nbt.1754.Integrative
Robinson JT, Thorvaldsdóttir H, Wenger AM, Zehir A, Mesirov JP (2017) Variant review with the integrative genomics viewer. Cancer Res 77(21):e31–e34. https://doi.org/10.1158/0008-5472.CAN-17-0337
Skalova A, Sar A, Laco J et al (2016) The Role of SATB2 as a diagnostic marker of sinonasal intestinal-type adenocarcinoma. Appl Immunohistochem Mol Morphol. https://doi.org/10.1097/PAI.0000000000000388(Epub ahea(00):1–7)
Szablewski V, Solassol J, Poizat F et al (2013) EGFR expression and KRAS and BRAF mutational status in intestinal-type sinonasal adenocarcinoma. Int J Mol Sci 14(3):5170–5181. https://doi.org/10.3390/ijms14035170
Tanaka K (2006) A case of metastases to the paranasal sinus from rectal mucinous adenocarcinoma. Int J Clin Oncol 11(1):64–65. https://doi.org/10.1007/s10147-005-0531-8
Thorvaldsdóttir H, Robinson JT, Mesirov JP (2013) Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief Bioinform 14(2):178–192. https://doi.org/10.1093/bib/bbs017
Thrumurthy SG, Thrumurthy SSD, Gilbert CE, Ross P, Haji A (2016) Colorectal adenocarcinoma: risks, prevention and diagnosis. BMJ 354:1–12. https://doi.org/10.1136/bmj.i3590
Tortora K, Vitali F, De Filippo C, Caderni G, Giovannelli L (2019) DNA damage in colon mucosa of Pirc rats, an Apc-driven model of colon tumorigenesis. Toxicol Lett 2020(324):12–19. https://doi.org/10.1016/j.toxlet.2020.02.002
Valente G, Ferrari L, Kerim S et al (2004) Evidence of p53 immunohistochemical overexpression in ethmoidal mucosa of woodworkers. Cancer Detect Prev 28(2):99–106. https://doi.org/10.1016/j.cdp.2003.12.004
Wang Z, Sun P, Gao C et al (2017) Down-regulation of LRP1B in colon cancer promoted the growth and migration of cancer cells. Exp Cell Res 357(1):1–8. https://doi.org/10.1016/j.yexcr.2017.04.010
Yom SS, Rashid A, Rosenthal DI et al (2005a) Genetic analysis of sinonasal adenocarcinoma phenotypes: distinct alterations of histogenetic significance. Mod Pathol 18(3):315–319. https://doi.org/10.1038/modpathol.3800315
Yom SS, Rashid A, Rosenthal DI et al (2005b) Genetic analysis of sinonasal adenocarcinoma phenotypes: distinct alterations of histogenetic significance. Mod Pathol Off J United States Can Acad Pathol Inc 18(3):315–319. https://doi.org/10.1038/modpathol.3800315
Zhang L, Shay JW (2017) Multiple roles of APC and its therapeutic implications in colorectal cancer. J Natl Cancer Inst 109(8):1–10. https://doi.org/10.1093/jnci/djw332
Funding
Sannia Mia Svenningsen Sjöstedt has received grants from King Christian XI Foundation and Harboe Foundation (both non-profit foundations) and Copenhagen University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted retrospectively using tissue samples obtained as part of routine diagnostics and treatment. Ethical approval was given by the Danish Regional Ethics Committee (H-17023164).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to incorrect reference number 30 (Hwang et al. 2019). The correct reference has been updated.
Rights and permissions
About this article
Cite this article
Sjöstedt, S., Schmidt, A.Y., Vieira, F.G. et al. Major driver mutations are shared between sinonasal intestinal-type adenocarcinoma and the morphologically identical colorectal adenocarcinoma. J Cancer Res Clin Oncol 147, 1019–1027 (2021). https://doi.org/10.1007/s00432-020-03421-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03421-5