Abstract
Purpose
The aim of this study was to determine the diagnostic yield of multigene panel testing among patients referred with hereditary breast and ovarian cancer (HBOC).
Methods
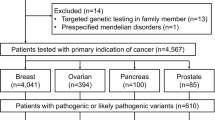
Patients who met provincial eligibility criteria were tested at the Advanced Molecular Diagnostic Laboratory at Mount Sinai Hospital, Toronto. Gene sequencing and exon-level copy number variant (CNV) analysis was performed. The referring physician had the opportunity to choose between several different gene panels based on patient phenotype. Cases were included in the analysis based on personal and family history of cancer and the type of panel ordered.
Results
3251 cases that received panel testing were included in this analysis. Overall, 9.1% (295) had a positive (pathogenic or likely pathogenic) result and 27.1% (882) had an inconclusive result (variant of uncertain significance). The genes with the highest prevalence of positive results were in BRCA2 (2.2%, 71/3235), BRCA1 (1.9%, 62/3235), and CHEK2 (1.4%, 40/2916). Of the positive cases, 9.8% (29) had a pathogenic or likely pathogenic variant in a gene associated with Lynch syndrome (MSH6, MSH2, MLH1, or PMS2).
Conclusions
Our overall positive yield is similar to that reported in the literature. The yield of inconclusive results was three times that of positive results. By testing more individuals in families with HBOC and through data-sharing efforts, the clinical significance of most variants may eventually be determined and panel testing for monogenic cancer predisposition syndromes will have greater utility.


Similar content being viewed by others
Data availability
Supporting data are available as supplementary materials.
Code availability
Not applicable.
Change history
09 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00432-020-03399-0
References
Burke W, Laberge AM, Press N (2010) Debating clinical utility. Public Health Genomics 13(4):215–223. https://doi.org/10.1159/000279623
Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL et al (2017) A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 123(10):1721–1730. https://doi.org/10.1002/cncr.30498
Cybulski C, Wokolorczyk D, Jakubowska A, Huzarski T, Byrski T, Gronwald J et al (2011) Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol 29(28):3747–3752. https://doi.org/10.1200/jco.2010.34.0778
Dossa F, Cusimano MC, Sutradhar R, Metcalfe K, Little T, Lerner-Ellis J et al (2018) Real-world health services utilisation and outcomes after BRCA1 and BRCA2 testing in Ontario, Canada: the What Comes Next Cohort Study protocol. BMJ Open 8(9):e025317. https://doi.org/10.1136/bmjopen-2018-025317
Feliubadalo L, Lopez-Fernandez A, Pineda M, Diez O, Del Valle J, Gutierrez-Enriquez S et al (2019) Opportunistic testing of BRCA1, BRCA2 and mismatch repair genes improves the yield of phenotype driven hereditary cancer gene panels. Int J Cancer 145(10):2682–2691. https://doi.org/10.1002/ijc.32304
Finch A, Wang M, Fine A, Atri L, Khalouei S, Pupavac M et al (2016) Genetic testing for BRCA1 and BRCA2 in the Province of Ontario. Clin Genet 89(3):304–311. https://doi.org/10.1111/cge.12647
Keeney MG, Couch FJ, Visscher DW, Lindor NM (2017) Non-BRCA familial breast cancer: review of reported pathology and molecular findings. Pathology 49(4):363–370. https://doi.org/10.1016/j.pathol.2017.03.002
Kohlmann, W., & Gruber, S. B. (2018). Lynch Syndrome. https://www.ncbi.nlm.nih.gov/books/NBK1211/
LaDuca H, Polley EC, Yussuf A, Hoang L, Gutierrez S, Hart SN et al (2020) A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med 22(2):407–415. https://doi.org/10.1038/s41436-019-0633-8
Lee K, Seifert BA, Shimelis H, Ghosh R, Crowley SB, Carter NJ et al (2018) Clinical validity assessment of genes frequently tested on hereditary breast and ovarian cancer susceptibility sequencing panels. Genet Med. https://doi.org/10.1038/s41436-018-0361-5
Lerner-Ellis J, Khalouei S, Sopik V, Narod SA (2015) Genetic risk assessment and prevention: the role of genetic testing panels in breast cancer. Expert Rev Anticancer Ther 15(11):1315–1326. https://doi.org/10.1586/14737140.2015.1090879
Lorans M, Dow E, Macrae F, Winship I, Buchanan D (2018) Update on hereditary colorectal cancer: improving the clinical utility of multigene panel testing. Clin Colorectal Cancer 17(2):e293–e305
Macklin S, Durand N, Atwal P, Hines S (2018) Observed frequency and challenges of variant reclassification in a hereditary cancer clinic. Genet Med 20(3):346–350. https://doi.org/10.1038/gim.2017.207
Mighton C, Charames G, Wang M, Zakoor K, Wong A, Shickh S et al (2019) Variant classification changes over time in BRCA1 and BRCA2. Genet Med 21(10):2248–2254
Nagy R, Sweet K, Eng C (2004) Highly penetrant hereditary cancer syndromes. Oncogene 23(38):6445–6470. https://doi.org/10.1038/sj.onc.1207714
National Collaborating Centre for Cancer (2013) National Institute for Health and Clinical Excellence: Guidance. In: Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. Cardiff: National Collaborating Centre for Cancer (UK)
National Comprehensive Cancer Network (2017) Genetic/familial high-risk assessment: breast and ovarian (version 2.2017). In: National Comprehensive Cancer Network.
Neben CL, Zimmer AD, Stedden W, van den Akker J, O'Connor R, Chan RC et al (2019) Multi-gene panel testing of 23,179 individuals for hereditary cancer risk identifies pathogenic variant carriers missed by current genetic testing guidelines. J Mol Diagn 21(4):646–657. https://doi.org/10.1016/j.jmoldx.2019.03.001
O'Leary E, Iacoboni D, Holle J, Michalski ST, Esplin ED, Yang S, Ouyang K (2017) Expanded gene panel use for women with breast cancer: identification and intervention beyond breast cancer risk. Ann Surg Oncol 24(10):3060–3066. https://doi.org/10.1245/s10434-017-5963-7
Petrucelli N, Daly M, Pal T (2016) BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. GeneReviews®. https://www.ncbi.nlm.nih.gov/books/NBK1247/
Piccinin C, Panchal S, Watkins N, Kim RH (2019) An update on genetic risk assessment and prevention: the role of genetic testing panels in breast cancer. Expert Rev Anticancer Ther 19(9):787–801. https://doi.org/10.1080/14737140.2019.1659730
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Shah PD, Nathanson KL (2017) Application of panel-based tests for inherited risk of cancer. Annu Rev Genomics Hum Genet 18:201–227. https://doi.org/10.1146/annurev-genom-091416-035305
Susswein LR, Marshall ML, Nusbaum R, Vogel Postula KJ, Weissman SM, Yackowski L et al (2016) Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet Med 18(8):823–832. https://doi.org/10.1038/gim.2015.166
Turner SA, Rao SK, Morgan RH, Vnencak-Jones CL, Wiesner GL (2018) The impact of variant classification on the clinical management of hereditary cancer syndromes. Genet Med. https://doi.org/10.1038/s41436-018-0063-z
Acknowledgements
This study was supported by the Ontario Ministry of Health. C.M. receives support from the Canadian Institutes of Health Research (GSD-164222). C.L. was a visiting scientist at Pathology and Laboratory Medicine, Mount Sinai Hospital and Women’s College Hospital thanks to Salvador Madariaga (PRX18/00267) and M-BAE (BA18/00018) grants (Spanish Government). We thank all of the clinicians who submitted the samples to our laboratory for testing. We would like to thank the laboratory staff who performed the work: E. Agro, A. Belay, E. Cox, S. Crafter, N. Di Nicola, K. Fenwick, K. Hamilton, A. Kiselova, G. Lee-Inniss, A. Lima Garay, J. Marr, J. Mayers, S. McArthur, A. Mitri, N. Moujani, O. Zeynep, S. Tancredi, K. Wagner, D. Yee, K.R. Zakoor, and M. Zhivotyagina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Ethical approval was obtained from the Research Ethics Board (REB) of Mount Sinai Hospital.
Consent for publication
All authors consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lerner-Ellis, J., Mighton, C., Lazaro, C. et al. Multigene panel testing for hereditary breast and ovarian cancer in the province of Ontario. J Cancer Res Clin Oncol 147, 871–879 (2021). https://doi.org/10.1007/s00432-020-03377-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03377-6