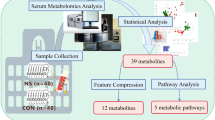
Abstract
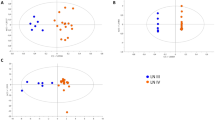
Henoch-Schönlein purpura nephritis (HSPN) is the most severe manifestation of Henoch-Schönlein purpura (HSP). This study aimed to determine the role of urine metabolomics in predicting HSPN and explore the potential mechanisms of HSP. A liquid chromatography-tandem mass spectrometry-based untargeted metabolomics analysis was performed to investigate the urinary metabolic profiles of 90 participants, comprising 30 healthy children (group CON) and 60 patients with HSP, including 30 HSP patients without renal involvement (group H) and 30 HSPN patients (group HSPN). The differentially expressed metabolites (DEMs) were identified using orthogonal partial least squares discriminant analysis (OPLS-DA), and subsequent bioinformatics analysis was conducted to elucidate the perturbed metabolic pathways. A total of 43 DEMs between H and HSPN groups were analyzed by the Kyoto Encyclopedia of Gene and Genome (KEGG) database, and the result indicates that glycine, serine and threonine metabolism, and cysteine and methionine metabolism were significantly disturbed. A composite model incorporating propionylcarnitine and indophenol sulfate was developed to assess the risk of renal involvement in pediatric patients with HSP.
Conclusion: This study reveals the metabolic alterations in healthy children, HSPN patients, and HSP patients without renal involvement. Furthermore, propionylcarnitine and indophenol sulfate may be potential predictive biomarkers of the occurrence of HSPN.
What is Known: • HSP is the predominant type of vasculitis observed in children. The long-term prognosis of HSP is contingent upon the extent of renal impairment. In severe nephritis, a delay in appropriate treatment may lead to fibrosis progression and subsequent development of chronic kidney disease (CKD), even leading to renal failure. • The application of metabolomics in investigating diverse renal disorders has been documented. Urine is a robust and sensitive medium for metabolomics detection. | |
What is New: • The metabolic profiles were identified in urine samples of healthy children and those with HSP at the early stage of the disease. Different metabolites were identified between HSP patients without nephritis and those who developed HSPN. • These different metabolites may affect oxidative stress in the progression of HSPN. |






Similar content being viewed by others
Availability of data and materials
All the data are available from the corresponding author upon reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- DEMs:
-
Differentially expressed metabolites
- ESI:
-
Electrospray ionization
- HMDB:
-
Human metabolome database
- HSP:
-
Henoch-Schönlein purpura
- HSPN:
-
Henoch-Schönlein purpura nephritis
- IgAV:
-
IgA vasculitis
- IgAVN:
-
IgA vasculitis nephritis
- IS:
-
Indoxyl sulfate
- KEGG:
-
Kyoto Encyclopedia of Gene and Genome
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- OPLS-DA:
-
Orthogonal partial least squares discriminant analysis
- PCA:
-
Principal component analysis
- ROC:
-
Receiver operating characteristic
- ROS:
-
Reactive oxygen species
References
Tadokoro T, Abe T, Nakano T, Kimura Y, Higaki K, Hayashidani S, Tashiro H (2023) IgA vasculitis. QJM 116:538–539. https://doi.org/10.1093/qjmed/hcad038
Chen JY, Mao JH (2015) Henoch-Schonlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr 11:29–34. https://doi.org/10.1007/s12519-014-0534-5
Nusken E, Weber LT (2022) IgA vasculitis nephritis. Curr Opin Pediatr 34:209–216. https://doi.org/10.1097/mop.0000000000001120
Rigante D, Candelli M, Federico G, Bartolozzi F, Porri MG, Stabile A (2005) Predictive factors of renal involvement or relapsing disease in children with Henoch-Schonlein purpura. Rheumatol Int 25:45–48. https://doi.org/10.1007/s00296-004-0452-2
Demir S, Kaplan O, Celebier M, Sag E, Bilginer Y, Lay I, Ozen S (2020) Predictive biomarkers of IgA vasculitis with nephritis by metabolomic analysis. Semin Arthritis Rheum 50:1238–1244. https://doi.org/10.1016/j.semarthrit.2020.09.006
Xu L, Li Y, Wu X (2022) IgA vasculitis update: epidemiology, pathogenesis, and biomarkers. Front Immunol 13:921864. https://doi.org/10.3389/fimmu.2022.921864
Song Y, Huang X, Yu G, Qiao J, Cheng J, Wu J, Chen J (2021) Pathogenesis of IgA vasculitis: an up-to-date review. Front Immunol 12:771619. https://doi.org/10.3389/fimmu.2021.771619
Rohner K, Marlais M, Ahn YH, Ali A, Alsharief A, Novak AB, Brambilla M et al (2024) Outcome of immunosuppression in children with IgA vasculitis–related nephritis. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfae009
Riccio S, Valentino MS, Passaro AP, Izzo M, Guarino S, Miraglia Del Giudice E, Marzuillo P, Di Sessa A (2022) New insights from metabolomics in pediatric renal diseases. Children (Basel). https://doi.org/10.3390/children9010118
Zhang Q, Lai LY, Cai YY, Wang MJ, Ma G, Qi LW, Xue J, Huang FQ (2021) Serum-urine matched metabolomics for predicting progression of Henoch-Schonlein Purpura nephritis. Front Med (Lausanne) 8:657073. https://doi.org/10.3389/fmed.2021.657073
Wen M, Dang X, Feng S, He Q, Li X, Liu T, He X (2021) Integrated analyses of gut microbiome and host metabolome in children with Henoch-Schonlein purpura. Front Cell Infect Microbiol 11:796410. https://doi.org/10.3389/fcimb.2021.796410
Gao Y (2021) On research and translation of urinary biomarkers. Adv Exp Med Biol 1306:101–108. https://doi.org/10.1007/978-3-030-63908-2_7
Chen J, Liu J, Cao D (2023) Urine metabolomics for assessing fertility-sparing treatment efficacy in endometrial cancer: a non-invasive approach using ultra-performance liquid chromatography mass spectrometry. BMC Womens Health 23:583. https://doi.org/10.1186/s12905-023-02730-4
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, Buoncompagni A et al (2010) EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteria. Ann Rheum Dis 69:798–806. https://doi.org/10.1136/ard.2009.116657
Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, Edworthy SM, Fauci AS, Leavitt RY, Lie JT (1990) The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum 33:1114–1121. https://doi.org/10.1002/art.1780330809
Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L et al (2012) A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol 30:918–920. https://doi.org/10.1038/nbt.2377
Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, Kanazawa M, VanderGheynst J, Fiehn O, Arita M (2015) MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods 12:523–526. https://doi.org/10.1038/nmeth.3393
Feng Q, Li Y, Yang Y, Feng J (2020) Urine metabolomics analysis in patients with normoalbuminuric diabetic kidney disease. Front Physiol 11:578799. https://doi.org/10.3389/fphys.2020.578799
Chasapi SA, Karagkouni E, Kalavrizioti D, Vamvakas S, Zompra A, Takis PG, Goumenos DS, Spyroulias GA (2022) NMR-based metabolomics in differential diagnosis of chronic kidney disease (CKD) subtypes. Metabolites. https://doi.org/10.3390/metabo12060490
Chiang FF, Chao TH, Huang SC, Cheng CH, Tseng YY, Huang YC (2022) Cysteine regulates oxidative stress and glutathione-related antioxidative capacity before and after colorectal tumor resection. Int J Mol Sci. https://doi.org/10.3390/ijms23179581
Xu S, Han S, Dai Y, Wang L, Zhang X, Ding Y (2022) A review of the mechanism of vascular endothelial injury in immunoglobulin A vasculitis. Front Physiol 13:833954. https://doi.org/10.3389/fphys.2022.833954
Mascolo E, Verni F (2020) Vitamin B6 and diabetes: relationship and molecular mechanisms. Int J Mol Sci. https://doi.org/10.3390/ijms21103669
Butbul Aviel Y, Dafna L, Pilar G, Brik R (2017) Endothelial function in children with a history of henoch schonlein purpura. Pediatr Rheumatol Online J 15:3. https://doi.org/10.1186/s12969-016-0135-z
Daiber A, Chlopicki S (2020) Revisiting pharmacology of oxidative stress and endothelial dysfunction in cardiovascular disease: evidence for redox-based therapies. Free Radic Biol Med 157:15–37. https://doi.org/10.1016/j.freeradbiomed.2020.02.026
Xie L, Liu Z, Lu H, Zhang W, Mi Q, Li X, Tang Y, Chen Q, Ferro A, Ji Y (2012) Pyridoxine inhibits endothelial NOS uncoupling induced by oxidized low-density lipoprotein via the PKCalpha signalling pathway in human umbilical vein endothelial cells. Br J Pharmacol 165:754–764. https://doi.org/10.1111/j.1476-5381.2011.01607.x
Ji Y, Diao J, Han Y, Huang Y, Bai H, Chen Q, Fan L, Ferro A (2006) Pyridoxine prevents dysfunction of endothelial cell nitric oxide production in response to low-density lipoprotein. Atherosclerosis 188:84–94. https://doi.org/10.1016/j.atherosclerosis.2005.10.035
Dalto DB, Matte JJ (2017) Pyridoxine (Vitamin B(6)) and the glutathione peroxidase system; a link between one-carbon metabolism and antioxidation. Nutrients. https://doi.org/10.3390/nu9030189
Finkelstein JD, Martin JJ, Harris BJ (1986) Effect of dietary cystine on methionine metabolism in rat liver. J Nutr 116:985–990. https://doi.org/10.1093/jn/116.6.985
Correia MJ, Pimpao AB, Fernandes DGF, Morello J, Sequeira CO, Calado J, Antunes AMM, Almeida MS, Branco P, Monteiro EC, Vicente JB, Serpa J, Pereira SA (2022) Cysteine as a multifaceted player in kidney, the cysteine-related thiolome and its implications for precision medicine. Molecules. https://doi.org/10.3390/molecules27041416
Pastore A, Noce A, Di Giovamberardino G, De Stefano A, Calla C, Zenobi R, Dessi M, Di Daniele N (2015) Homocysteine, cysteine, folate and vitamin B(1)(2) status in type 2 diabetic patients with chronic kidney disease. J Nephrol 28:571–576. https://doi.org/10.1007/s40620-014-0126-4
Sarnak MJ, Wang SR, Beck GJ, Kusek JW, Selhub J, Greene T, Levey AS (2002) Homocysteine, cysteine, and B vitamins as predictors of kidney disease progression. Am J Kidney Dis 40:932–939. https://doi.org/10.1053/ajkd.2002.36323
Yu R, Wu Y, He P, Bai Y, Zhang Y, Bian X, Sun G, Zhang B (2023) LIM and cysteine-rich domains 1 promotes transforming growth factor beta1-induced epithelial-mesenchymal transition in human kidney 2 cells. Lab Invest 103:100016. https://doi.org/10.1016/j.labinv.2022.100016
Suljevic D, Mitrasinovic-Brulic M, Focak M (2023) L-cysteine protective effects against platelet disaggregation and echinocyte occurrence in gentamicin-induced kidney injury. Mol Cell Biochem 478:13–22. https://doi.org/10.1007/s11010-022-04498-x
Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work Group (2012) Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23:1258–1270. https://doi.org/10.1681/asn.2011121175
Gao H, Liu S (2017) Role of uremic toxin indoxyl sulfate in the progression of cardiovascular disease. Life Sci 185:23–29. https://doi.org/10.1016/j.lfs.2017.07.027
Cheng TH, Ma MC, Liao MT, Zheng CM, Lu KC, Liao CH, Hou YC, Liu WC, Lu CL (2020) Indoxyl sulfate, a tubular toxin, contributes to the development of chronic kidney disease. Toxins (Basel). https://doi.org/10.3390/toxins12110684
Saigo C, Nomura Y, Yamamoto Y, Sagata M, Matsunaga R, Jono H, Nishi K, Saito H (2014) Meclofenamate elicits a nephropreventing effect in a rat model of ischemic acute kidney injury by suppressing indoxyl sulfate production and restoring renal organic anion transporters. Drug Des Devel Ther 8:1073–1082. https://doi.org/10.2147/dddt.s67456
Yu M, Kim YJ, Kang DH (2011) Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol 6:30–39. https://doi.org/10.2215/cjn.05340610
Dias GF, Bonan NB, Steiner TM, Tozoni SS, Rodrigues S, Nakao LS, Kuntsevich V, Pecoits Filho R, Kotanko P, Moreno-Amaral AN (2018) Indoxyl sulfate, a uremic toxin, stimulates reactive oxygen species production and erythrocyte cell death supposedly by an organic anion transporter 2 (OAT2) and NADPH oxidase activity-dependent pathways. Toxins (Basel). https://doi.org/10.3390/toxins10070280
Makarova E, Makrecka-Kuka M, Vilks K, Volska K, Sevostjanovs E, Grinberga S, Zarkova-Malkova O, Dambrova M, Liepinsh E (2019) Decreases in circulating concentrations of long-chain acylcarnitines and free fatty acids during the glucose tolerance test represent tissue-specific insulin sensitivity. Front Endocrinol (Lausanne) 10:870. https://doi.org/10.3389/fendo.2019.00870
Dambrova M, Makrecka-Kuka M, Kuka J, Vilskersts R, Nordberg D, Attwood MM, Smesny S, Sen ZD, Guo AC, Oler E, Tian S, Zheng J, Wishart DS, Liepinsh E, Schioth HB (2022) Acylcarnitines: nomenclature, biomarkers, therapeutic potential, drug targets, and clinical trials. Pharmacol Rev 74:506–551. https://doi.org/10.1124/pharmrev.121.000408
Libert DM, Nowacki AS, Natowicz MR (2018) Metabolomic analysis of obesity, metabolic syndrome, and type 2 diabetes: amino acid and acylcarnitine levels change along a spectrum of metabolic wellness. PeerJ 6:e5410. https://doi.org/10.7717/peerj.5410
Manoli I, Gebremariam A, McCoy S, Pass AR, Gagne J, Hall C, Ferry S, Van Ryzin C, Sloan JL, Sacchetti E, Catesini G, Rizzo C, Martinelli D, Spada M, Dionisi-Vici C, Venditti CP (2023) Biomarkers to predict disease progression and therapeutic response in isolated methylmalonic acidemia. J Inherit Metab Dis 46:554–572. https://doi.org/10.1002/jimd.12636
Acknowledgements
The authors wish to extend their appreciation to the children and their families and the personnel at the health physical examination center of the Children’s Hospital of Soochow University for their assistance and support in participant recruitment.
Funding
The study was supported by the Medical Research Project of the Jiangsu Commission of Health (K2023049).
Author information
Authors and Affiliations
Contributions
MY, XS, and JT were responsible for study conceptualization. JG collected the urine samples and the clinical data. MY and XS generated data, analyzed results, and drafted the manuscript. QF and JT reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the Children’s Hospital of Soochow University (2023CS180) and conducted under the Declaration of Helsinki. Informed consent was obtained from all patients’ parents.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, M., Song, X., Guo, J. et al. Exploring potential predictors of Henoch-Schönlein purpura nephritis: a pilot investigation on urinary metabolites. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05573-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05573-9