Abstract
This review summarizes current knowledge on post-acute sequelae of COVID-19 (PASC) and post-COVID-19 condition (PCC) in children and adolescents. A literature review was performed to synthesize information from clinical studies, expert opinions, and guidelines. PASC also termed Long COVID — at any age comprise a plethora of unspecific symptoms present later than 4 weeks after confirmed or probable infection with severe respiratory syndrome corona virus type 2 (SARS-CoV-2), without another medical explanation. PCC in children and adolescents was defined by the WHO as PASC occurring within 3 months of acute coronavirus disease 2019 (COVID-19), lasting at least 2 months, and limiting daily activities. Pediatric PASC mostly manifest after mild courses of COVID-19 and in the majority of cases remit after few months. However, symptoms can last for more than 1 year and may result in significant disability. Frequent symptoms include fatigue, exertion intolerance, and anxiety. Some patients present with postural tachycardia syndrome (PoTS), and a small number of cases fulfill the clinical criteria of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). To date, no diagnostic marker has been established, and differential diagnostics remains challenging. Therapeutic approaches include appropriate self-management as well as the palliation of symptoms by non-pharmaceutical and pharmaceutical strategies.
Conclusion: PASC in pediatrics present with heterogenous severity and duration. A stepped, interdisciplinary, and individualized approach is essential for appropriate clinical management. Current health care structures have to be adapted, and research was extended to meet the medical and psychosocial needs of young people with PASC or similar conditions.
What is Known: • Post-acute sequelae of coronavirus 2019 (COVID-19) (PASC) — also termed Long COVID — in children and adolescents can lead to activity limitation and reduced quality of life. • PASC belongs to a large group of similar post-acute infection syndromes (PAIS). Specific biomarkers and causal treatment options are not yet available. | |
What is New: • In February 2023, a case definition for post COVID-19 condition (PCC) in children and adolescents was provided by the World Health Organization (WHO), indicating PASC with duration of at least 2 months and limitation of daily activities. PCC can present as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). • Interdisciplinary collaborations are necessary and have been established worldwide to offer harmonized, multimodal approaches to diagnosis and management of PASC/PCC in children and adolescents. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shortly after the onset of the pandemic, the term Long COVID had been introduced by people experiencing a broad variety of persisting symptoms following the infection with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) [1]. Long-term symptoms were described after any clinical course of the initial SARS-CoV-2 infection and at any age, with impairment of many people’s daily activities and health-related quality of life (HRQoL) [2]. The umbrella terms Long COVID and post-acute sequelae of COVID-19 (PASC) are mostly being used for SARS-CoV-2-associated symptoms later than 4 weeks [3], and “Post-COVID-19 Syndrome” (PCS) for symptoms later than 12 weeks after the initial infection [4, 5]. A post-COVID-19 condition (PCC) was defined by the WHO in October 2021 as the continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months and no other explanation [6]. To indicate PCC, the assign code U09.9! was added to the 10th Revision of International Classification of Diseases internationally (ICD-10) [7, 8]. However, no PCC case definition was provided for children until February 2023 [6], and evidence from pediatric research still is not conclusive regarding many features of PASC and PCC [9, 10]. This is due to the large heterogeneity of study designs, the paucity of studies including uninfected control groups, and the limited representativity of studies from distinct countries due to different courses of the pandemic and different strategies of its management worldwide [9, 10]. This review aims at summarizing current clinical perspectives on PASC and similar disorders.
Materials and methods
A literature search was conducted in PubMed between March 20, 2023, and June 20, 2023, with search terms combined by Boolean operators (AND, OR) and truncated search terms according to the PubMed User Guide. PubMed’s Automatic Term Mapping was applied as well as the following MESH terms used: (Long COVID) OR (Post-COVID) OR (Post COVID) OR (post-acute AND COVID) OR (post-acute AND SARS*) OR (sequelae AND COVID) OR (sequelae AND SARS*) OR (ME/CFS) OR (chronic fatigue syndrome) AND (pediatr*) OR (paediatr*) OR (child*) OR (kid*) OR (infant*) OR (toddler*) OR (pre-schooler*) OR (preschooler*) OR (adolescen*) OR (youth*) OR (teen*). Additional literature was added based on non-systematical online search in PubMed and on preprint servers as well es in the ClinicalTrials.gov Protocol Registration and Results System. To narrow the search results, the primary review inclusion criteria were applied using the following filters: species = human, language = English, and patients below 18 years of age. Evidence from studies with adult patients was discussed and added when appropriate. The final decision to include studies into the review was consented by all authors.
Results
Definition
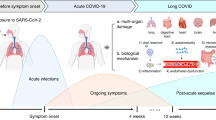
In addition to earlier definitions for PASC and PCS at any age [11, 12], a pediatric research definition was suggested for Long COVID/PCC by a modified Delphi process in 2022 [13]. In February 2023, a clinical case definition of a PCC in children was provided by the WHO [14]. According to this definition, PCC “occurs in individuals with a history of confirmed or probable SARS-CoV-2 infection, when experiencing symptoms lasting at least two months which initially occurred within three months of acute COVID” (Fig. 1). The WHO stated that “symptoms generally have an impact on everyday functioning such as changes in eating habits, physical activity, behavior, academic performance, social functions (..) and developmental milestones” and, moreover, “may be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness” and “may also fluctuate or relapse over time.” Whether a SARS-CoV-2-triggered exacerbation or aggravation of pre-existing morbidities (e.g., bronchial asthma, migraine, and depression) should be excluded from the PCC definition at any age is still a matter of debate [15].
In addition to earlier definitions for long-term sequelae of coronavirus 2019 (COVID-19), a pediatric definition for a post-COVID condition (PCC) was suggested by a modified Delphi process within the CloCk consortium (CLoCk) in July 2022 for research purposes [13] and by the World Health Organization (WHO) in February 2023 for clinical use [14]. Definitions were as follows: 1Post COVID-19 condition (PCC, WHO), “in children and adolescents occurs in individuals with a history of confirmed or probable SARS-CoV-2 infection, when experiencing symptoms lasting at least 2 months which initially occurred within 3 months of acute COVID-19... Symptoms generally have an impact on everyday functioning... Symptoms may be new onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. They may also fluctuate or relapse over time. Workup may reveal additional diagnoses, but this does not exclude the diagnosis of post COVID-19 condition. This can be applied to children of all ages, with age-specific symptoms and impact on everyday function taken into consideration”. (2/2023) 2Post-COVID condition in children (CLoCk), “occurs in young people with a history of confirmed SARS-CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID infection, and may fluctuate or relapse over time. The positive COVID-19 test referred to in this definition can be a lateral flow antigen test, a PCR test or an antibody test”. (7/2022)
Clinical features
PASC present with a variety of unspecific symptoms [14] and significant inter-individual heterogeneity at any age [16]. Fatigue, altered smell or anosmia, and anxiety were pointed out by the WHO as symptoms most strongly associated with PCC in children and adolescents, with 21 additional symptoms listed, including chest pain, cognitive difficulties, cough, diarrhea, dizziness, dyspnea, earache/ringing in ears, fever, headache, insomnia, joint pain or swelling, light sensitivity, loss of appetite, mood swings, myalgia, nausea, palpitations, postural symptoms, rash, stomach ache, and sore eyes or throat [14, 17]. Like in adults, “brain fog” which comprises various cognitive impairment sequelae is reported by adolescents and children [17, 18]. Fatigue was identified as one of the most frequent symptoms of PASC at any age in many studies [19, 20] and together with “post-exertional malaise” (PEM) represents the cardinal symptoms of ME/CFS (ICD-10 G93.3) [21,22,23]. PEM is frequent in PASC as well and was termed post-exertional symptom exacerbation (PESE) or post-exertional neuroimmune exhaustion (PENE) in some studies [24,25,26,27]. It is defined as a worsening of symptoms after daily activities that were well-tolerated before, often manifests only 12 to 48 h after activity, and can last for days or even weeks [28].
ME/CFS was documented as severe subtype of PCC in adults [29, 30] and was recently reported by our pediatric group in patients with PCC younger than 18 years [31]. ME/CFS is associated with a HRQoL lower than in other chronic diseases, and significantly impairs social participation in most cases [21, 22, 32]. The clinical features of PASC resemble those of other post-acute infection syndromes (PAIS). PAIS had been described for decades at any age, including ME/CFS [33,34,35,36,37]. PAIS can occur after infection with viruses, bacteria, and protozoa, with EBV representing a prominent trigger in adolescents and children [38, 39]. Importantly, post-acute vaccination syndromes (PAVS) with similar clinical features have recently been reported in adults, adolescents, and children after COVID-vaccination (post-COVIDvac/Post-Vac-syndrome) [40, 41].
Epidemiology
Since the emergence of the SARS-CoV-2 variant omicron (B.1.1.529), the COVID-19 pandemic has gradually evolved into an endemic situation. Meanwhile, most adolescents and children had one or more contacts with SARS-CoV-2 and/or its spike antigen included in COVID-19 vaccines [42, 43]. With the increasing probability of SARS-CoV-2 infection in history, reduced SARS-CoV-2 testing worldwide, and non-persistence of SARS-CoV-2 antibodies in many individuals, a clear attribution of persisting or new symptoms to a previous SARS-CoV-2 infection is not possible in many cases. However, the lack of a documented specific infection is frequent in PAIS, and the diagnosis, in these cases, has to be based on a probable initial infection. This diagnostic challenge is well known in pediatrics from PAIS after Epstein-Barr virus (EBV)-associated infectious mononucleosis [17, 39, 44].
The prevalences of PASC and PCC as well as subgroups thereof in the pediatric age group are still largely unknown. PASC was reported with 25% in a large pediatric meta-analysis [9]. An umbrella review of PCC in initially non-hospitalized children found a prevalence of 2.0–3.5% [45]. The pooled prevalence of PCC symptoms in studies with a supposedly SARS-CoV-2-negative control group was lower than in uncontrolled studies [46]. Controlled studies reported a range from less than 1% to more than 40%, with less than 5% in most studies. A final conclusion on prevalence is difficult due to the heterogeneity regarding definitions as well as addressed cohorts (e.g., populations-based, hospital-based) and investigated symptoms. Data were collected from self-reports in many studies, and controls were selected by different measures [9, 10, 45,46,47].
Pathogenesis and risk factors
Several pathogenic mechanisms have been suggested for PASC and other PAIS. These include persistence of virus and/or viral components, virus-induced tissue damage, endothelial dysfunction, coagulopathy, autonomic dysfunction, chronic inflammation, and autoimmunity [35, 36, 47,48,49,50,51]. The risk of developing PASC is two to fourfold higher after infection with a pre-omicron variant [52, 53], and COVID-19 vaccination was shown to reduce the risk of PASC, depending on the triggering virus variant [53,54,55,56]. Moreover, higher age, female gender, and pre-existing health issues were suggested to increase the risk in the pediatric population [9, 46, 47, 57, 58]. A score was suggested for risk calculation in pediatrics, including SARS-CoV-2 status, number of symptoms at testing, sex, age, ethnicity, physical and mental health, loneliness, and four EQ-5D-Y scale items (problems looking after self, doing usual activities, having pain, and feeling worried/sad) before testing [59]. Immunological genetic variants are addressed as candidate risk factors in ongoing studies [60]. However, risk factors for severe compared to mild and moderate PASC subtypes are largely unknown.
Diagnostics
No biomarker is available yet for the diagnosis of PASC which therefore has to be differentiated from other medical conditions that may elicit similar symptoms, including other PAIS. Of 110 pediatric patients with suspected PASC admitted to a specialized outpatient clinic, only 29% received a final PASC diagnosis while 47% were diagnosed with alternative somatic/mental diseases, and 23% with diagnosis unclarified [61]. Differential diagnoses include a broad variety of somatic (e.g., pulmonary, cardiovascular, neurological, rheumatic, oncological, gastrointestinal, and metabolic) and psychiatric (e.g., depression, anxiety, attention deficit (hyperactivity), somatization, conversion, and eating) disorders as well as mental distress due to social distancing or loss of relatives during the pandemic (“long-lockdown,” “post-pandemic-syndrome”). Racine et al. reported almost a doubling of the global prevalence of depressive and anxiety symptoms in children and adolescents during the pandemic as compared to before [62]. In Germany, although mental health of the youth improved in year three of the pandemic, it is still lower than pre-pandemic [63]. Accordingly, many studies revealed high loads of unspecific symptoms in both SARS-COV-2-infected cases and SARS-COV-2-non-infected controls. For example, 1560 students recruited from Dresden schools with or without detectable anti-SARS-CoV-2 antibodies in 2020/21 showed similar rates of concentration problems, listlessness, fatigue, and mood swings (40–80%) [64]. The LongCOVIDKidsDK study found 46% and 41% adolescents with or without a positive SARS-CoV-2 test in history reporting PCC-like symptoms, respectively [65]. In the CLoCk Study, 24.5% and 17.8% of initially SARS-CoV-2 test-positive and SARS-CoV-2 test-negative children and adolescents had PCC-like symptoms at 6 months [66]. Furthermore, somatic and especially psychiatric comorbidities have to be carefully taken into account. Thus, PASC/PCC-like symptoms are challenging both clinical diagnostics as well as epidemiological studies, especially in children.
Therefore, in order to ensure a thorough diagnostic assessment, it should be aimed to evaluate patients simultaneously by a pediatrician and child and adolescent psychiatrist or psychologist. Algorithms have been published for adults, adolescents, and children with PASC to allow for a stepped and individualized diagnostic approach [4, 5, 18]. Diagnostics include a thorough medical and psychosocial history, usually in form of a semi-structured qualitative interview, a comprehensive physical exam and psychological assessment, as well as routine blood analyses, supplemented by function tests, neuropsychological testing, and/or imaging, depending on the individual symptoms. Basic blood analyses should address differential cell count, C-reactive protein (CRP), liver, renal, and thyroid function, glucose, as well as anti-nuclear and anti-transglutaminase antibodies. Endocrinopathies can manifest in the context of COVID-19 and therefore need special attention [67, 68]. Basic function tests comprise Holter electrocardiogram, pulmonary function test, passive standing test [69], 6-min walk test, and/or 1-min sit-to-stand test. The passive standing test (or head-up tilt table test) aims at detecting postural tachycardia syndrome (PoTS) in patients with a history of orthostatic symptoms. PoTS is defined by a sustained heart rate ≥ 120 beats per minute (bpm) and/or a sustained heart rate increase by ≥ 40 bpm within 10 min standing compared to supine for individuals ≤ 19 years [4, 5, 47, 70]. Supplementary tests might include an electroencephalogram, cardiac and/or lung ultrasound, chest X-ray, computer tomography, magnet resonance imaging (MRI), cardiopulmonary exercise testing (CPET) and/or ventilation–perfusion single-photon emission computed tomography (V/Q SPECT), and neurocognitive testing. Both SPECT/CT and CPET suggested pulmonary circulation dysfunction in a 13-year-old child [71]. However, low-field-strength MRI showed persistent pulmonary dysfunction in both pediatric patients who recovered from COVID-19 and those with PASC [72]. With regard to neurocognitive testing so far, there is no PASC specific test battery available, and pediatric data mainly lacking. It might be challenging to select appropriate diagnostic measures, and patients with severe PEM might not tolerate the full routine work-up.
Early identification of PEM is important to both stratifying diagnostics and management. In order to detect PEM, the brief DePaul Symptom Questionnaire (DSQ) was developed which addresses the severity, frequency, and duration of PEM symptoms at any age. It correctly categorized adult patients with ME/CFS 81.7% of the time, while incorrectly categorizing multiple sclerosis (MS) and post-polio syndrome as ME/CFS only 16.6% of the time [73]. We have used the DSQ-PEM together with our novel Munich Berlin Symptom Questionnaire (MBSQ) to identify PEM and ME/CFS in children, adolescents, and adults with PCC, respectively [31]. Semi-structured qualitative interviews before and after CPET in adults indicated that patients had unique PEM experiences, with differences regarding onset, severity, trajectory over time, and most bothersome symptoms [74]. However, little is known on PCC-PEM in children and adolescents, and CPET is not applicable to routine diagnostics of people with probable PEM due to the risk of worsening the symptoms.
Management
No specific causal treatment was established yet for PAIS, including PASC and ME/CFS. However, all patients should be offered appropriate holistic care, including non-pharmaceutical and pharmaceutical approaches as individually needed. The most bothersome symptoms should be prioritized, and multi-modal, multi-professional strategies are developed for those with complex and/or severe symptoms [4, 5, 18, 47, 70, 75].
As a first step, disease-specific education should be provided to patients and parents about current knowledge, remaining uncertainties, and patient advocacy groups. An introduction to self-management strategies [4, 5, 18, 47, 70, 75] should address relaxation techniques, deep breathing exercises, sleep hygiene, gentle muscle training if tolerated, coping strategies, as well as a the 3P energy conservation strategy (prioritizing, planning, and pacing) for patients with PEM [4, 5, 75]. The pacing means to be as active as possible within the individual limits, without inducing PEM [76, 77].
Depending on the individual needs, non-pharmaceutical options of care also include physiotherapy, respiratory therapy, psychotherapy, cognitive training, occupational therapy, and transcutaneous electrical nerve stimulation (TENS), as well as the prescription of medical aids (e.g., compression stockings, wheel chair), appropriate social support (e.g., certificates for schools, applying for an appropriate grade of disability), and home care in severe cases [4, 5, 47, 70].
Pharmaceutical treatment should follow current guidelines (e.g., for pain, sleeping disorders, migraine), and deficits of vitamins or minerals should be supplemented [4, 5, 18, 47, 70]. However, off-label use of drugs which are approved for other indications might help in some cases (e.g., for PoTS or “brain fog”). Ideally this would occur after consulting specialists with pediatric expertise in treating PAIS and/or ME/CFS, recognizing that these resources might not be available in all locations. Patients with bronchial hyperresponsiveness may benefit from inhaled corticosteroids [35]. Orthostatic intolerance may improve with adequate salt and fluid intake, compression stockings, gentle exercises, as well as additional measures to modulate heart rate (e.g., ivabradine) and/or stabilize blood pressure (e.g., midodrine). Up to now, anticoagulation without clear evidence of a coagulation disorder is not indicated [36].
In adults, several randomized controlled trials (RCT) are investigating pharmaceutical approaches to PCC, including antiviral drugs as well as immunomodulatory, neuromodulatory, and vasoactive substances [78]. A prominent approach is the depletion of pathogenic autoantibodies, e.g., by plasma exchange, immunoadsorption, or infusion of the aptamer BC007 [79]. Hyperbaric oxygen therapy (HBOT) was shown to improve neurocognitive function in a phase II RCT for PCC [80] and will be further investigated within our National Clinical Study Group on PCC and ME/CFS (NKSG) [78]. We are not aware of any published results from interventional trials addressing PSC in children or adolescents. The website ClinicalTrials.gov lists an ongoing phase 2a randomized, double-blind, placebo-controlled clinical trial evaluating larazotide (AT1001) [81] and a double-blind, controlled trial investigating vitamin D [82].
Prognosis
For the majority of children and adolescents with PASC, the prognosis is favorable, and symptoms decline within 6 months [83, 84]. Accordingly, several pre-pandemic studies indicated that a significant number of children and adolescents with PAIS, including ME/CFS following primary EBV infection, recovered [32, 38, 44]. A pediatric long-term follow-up study of ME/CFS reported that “paying attention to social learning needs and educational support have been identified by young people as being of similar importance to medical management” [32].
Limited social participation and school absenteeism
Many students with PAIS, including PASC and ME/CFS, have to limit or omit sports and/or after-school activities, and some might not be able to attend school full- or part-time. Pre-pandemic ME/CFS was identified as the most common cause of long-term school absence [85,86,87,88,89]. However, the effect of PASC on school performance is not well studied yet [90]. Remarkably, a recent survey from Germany reported a 38% increase of general school absenteeism compared to pre-pandemic years [38], in Belgium school absenteeism in secondary education also increased by 56% between 2020/2021 and 2021/2022 [91]. Early detection of school absenteeism, education of school staff about PAIS and PEM, as well as appropriate diagnostics to differentiate between somatic, psychiatric, and social causes seem increasingly important.
Stigmatization and disbelieve
Many patients with severe PAIS still are facing inadequate medical support, stigmatization, and disbelieve [42, 89, 90, 92, 93]. Parents of patients with PASC reported on “medical gaslighting” or “trivialization” of their concerns via social media [94] and asked for treatment with “compassion” [95]. A survey with adult PACS patients indicated that they were “encountering medical professionals who dismissed their experience, leading to lengthy diagnostic odysseys and lack of treatment options” [96]. Accordingly, current surveys indicate that patients with pre-pandemic ME/CFS and patients with PASC seek mutual support [97]. Parent advocacy groups such as “Long COVID KIDS” [98] and others [99, 100] might be helpful to families burdened by PAIS in children and adolescents.
Discussion
Stepped approaches to individualized, holistic care
PAIS, including PASC and ME/CFS, are complex disorders with non-specific symptoms affording appropriate differential diagnosis and multi-modal, multi-professional treatment in many cases. Primary physicians may initiate first steps to diagnosis, with following steps depending on the severity and duration of the individual illness. Cases with significant loss of participation should be prioritized for admission to specialists. First PCC and ME/CFS competence centers have been implemented, providing complex patient care as well as the transfer of knowledge and research. At all steps of patient care, a holistic, individualized approach is recommended, and care should be offered to patients with PAIS and PAVS [4, 5, 47, 70]. However, so far, the number of specialized institutions in Germany and Europe does not meet the urgent needs, and budgets provided by health insurances currently do not cover expenses at any level of sector-spanning, stepped medical care.
Challenges of post-exertional malaise
Exertion intolerance with PEM is a challenging current health care concept. Affected patients might not be able to tolerate routine diagnostics and be unable to follow all therapeutic recommendations. Medical procedures have to be adapted to allow for breaks, to shorten distances, to reduce light and noises, and to prioritize the most bothering problems. This is true for the setting of the primary physician as well as for secondary and tertiary health care institutions, including rehabilitation facilities. Some patients with PEM or ME/CFS are not mobile enough to visit any medical institution and need multi-professional, multi-modal home treatment, supported by telemedical advice from specialists [4, 5, 18]. However, options for adequate multi-professional rehabilitation, home care, and telemedicine are not available yet as needed, and strategies have to be developed to protect affected patients from stigmatization and inadequate treatment. Because orthostatic stress can aggravate symptoms of ME/CFS and PASC/PCC, it is reasonable to propose that treating orthostatic stress has the potential to help with managing PEM, although more research on this topic is needed [101,102,103,104,105,106].
Core outcome sets and future research
Clinical features and pathomechanisms of PAIS are increasingly addressed in pediatric studies, including ours [107,108,109,110,111]. However, a lack of harmonization limits the comparability worldwide. A library of common data elements was implemented before the pandemic in the USA to streamline research on ME/CFS [112], and a core outcome set (COS) for PCC in adults and children were recently agreed upon [113] and initiated [114], respectively. International collaborations on PAIS and ME/CFS are urgently needed to fill the gap of pediatric data and thereby pave the way to optimal patient care and prevention.
Data availability
As all data included in this review are already available via the cited references, a special statement on data availability is not applicable for this article.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- CPET:
-
Cardiopulmonary exercise testing
- DSQ:
-
DePaul Symptom Questionnaire
- EBV:
-
Epstein-Barr virus
- HBOT:
-
Hyperbaric oxygen therapy
- HRQoL:
-
Health-related quality of life
- ICD-10-GM:
-
10Th Revision of the International Classification of Diseases–German Modifications
- MBSQ:
-
Munich Berlin Symptom Questionnaire
- ME/CFS:
-
Myalgic encephalomyelitis/chronic fatigue syndrome
- MIS-C:
-
Multisystem inflammatory syndrome in children
- MRI:
-
Magnetic resonance imaging
- MS:
-
Multiple sclerosis
- NKSG:
-
National Clinical Study Group
- PACS:
-
Post-acute COVID-19 sequelae
- PAVS:
-
Post-acute vaccination syndromes
- PCC:
-
Post-COVID-19 condition
- PCS:
-
Post-COVID syndrome
- PEM:
-
Post-exertional malaise
- PENE:
-
Post-exertional neuroimmune exhaustion
- PESE:
-
Post-exertional symptom exacerbation
- PICS:
-
Post-intensive care syndrome
- PIMS:
-
Pediatric inflammatory multisystem syndrome
- PoTS:
-
Postural tachycardia syndrome
- RCT:
-
Randomized controlled trial
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus type 2
- SPC:
-
Social Pediatrics Center
- SPECT/CT:
-
Single-photon emission computed tomography
- TENS:
-
Transcutaneous electrical nerve stimulation
- V/Q SPECT:
-
Ventilation–perfusion single photon emission computed tomography
- WHO:
-
World Health Organization
References
Alwan NA (2020) A negative COVID-19 test does not mean recovery. Nature 584:170–170. https://doi.org/10.1038/d41586-020-02335-z
Nalbandian A, Sehgal K, Gupta A et al (2021) Post-acute COVID-19 syndrome. Nat Med 27:601–615. https://doi.org/10.1038/s41591-021-01283-z
Groff D, Sun A, Ssentongo AE et al (2021) Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 4:1–17. https://doi.org/10.1001/jamanetworkopen.2021.28568
Töpfner N, Alberer M, Ankermann T et al (2022) Einheitliche Basisversorgung von Kindern und Jugendlichen mit Long COVID. Monatsschrift Kinderheilkunde 170:539–547. https://doi.org/10.1007/s00112-021-01408-1
Koczulla AR, Ankermann T, Behrends U et al (2022) S1-Leitlinie long-/post-COVID. Pneumologie 76:855–907. https://doi.org/10.1055/a-1946-3230
World Health Organization (WHO) (2021) A clinical case definition of post COVID-19 condition by a Delphi consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1
Centers for Disease Control and Prevention (CDC) (2021) New ICD-10-CM code for post-COVID conditions, following the 2019 novel coronavirus (COVID-19). https://www.cdc.gov/nchs/data/icd/announcement-new-icd-code-for-post-covid-condition-april-2022-final.pdf
Bundesinstitut für Arzneimittel und Medizinprodukte (DIMDI) (2021) ICD-10-GM Version 2021. Kapitel XXII Schlüsselnummern für besondere Zwecke (U00-U99). https://www.dimdi.de/static/de/klassifikationen/icd/icd-10-gm/kode-suche/htmlgm2021/block-u00-u49.htm
Pellegrino R, Chiappini E, Licari A et al (2022) Prevalence and clinical presentation of long COVID in children: a systematic review. Eur J Pediatr 181:3995–4009. https://doi.org/10.1007/s00431-022-04600-x
Zimmermann P, Pittet LF, Curtis N (2022) The challenge of studying long COVID: an updated review. J Pediatr Infect Dis 41:424–426. https://doi.org/10.1097/INF.0000000000003502
Koczulla AR, Ankermann T, Behrends U, Berlit P, Berner R, Böing S, Brinkmann F, Frank U, Franke C, Glöckl R, Gogoll C (2022) S1-Leitlinie long/post-COVID - living guideline. AWMF online: Das Portal der wissenschaftichen Medizin. https://register.awmf.org/de/leitlinien/detail/020-027
National Institute for Health and Care Excellence (NICE) (2020) COVID-19 rapid guideline: managing the long-term effects of COVID-19, NICE guideline [NG188]. Last updated: 11 November 2021. https://www.nice.org.uk/guidance/ng188
Stephenson T, Allin B, Nugawela MD et al (2022) Long COVID (post-COVID-19 condition) in children: a modified Delphi process. Arch Dis Child 107:674–680. https://doi.org/10.1136/archdischild-2021-323624
World Health Organization (WHO) (2023) A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1
Hallek M, Adorjan K, Behrends U et al (2023) Post-COVID syndrome. Dtsch Arztebl Int. https://doi.org/10.3238/arztebl.m2022.0409
Stephenson T, Shafran R, Ladhani SN (2022) Long COVID in children and adolescents. Curr Opin Infect Dis 35:461–467. https://doi.org/10.1097/QCO.0000000000000854
Lopez-Leon S, Wegman-Ostrosky T, Perelman C et al (2021) More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 11:16144. https://doi.org/10.1038/s41598-021-95565-8
Ha EK, Kim JH, Han MY (2023) Long COVID in children and adolescents: prevalence, clinical manifestations, and management strategies. Clin Exp Pediatr. https://doi.org/10.3345/cep.2023.00472
Davis HE, McCorkell L, Vogel JM, Topol EJ (2023) Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 21:133–146. https://doi.org/10.1038/s41579-022-00846-2
Roessler M, Tesch F, Batram M et al (2022) Post-COVID-19-associated morbidity in children, adolescents, and adults: a matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med 19:e1004122. https://doi.org/10.1371/journal.pmed.1004122
Nacul L, Authier FJ, Scheibenbogen C et al (2021) European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Medicina 57:510. https://doi.org/10.3390/medicina57050510
Rowe PC, Underhill RA, Friedman KJ et al (2017) Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: a primer. Front Pedia 5. https://doi.org/10.3389/fped.2017.00121
Centers for Disease Control and Prevention (CDC) Myalgic encephalomyelitis/chronic fatigue syndrome. Symptoms and diagnosis of ME/CFS. https://www.cdc.gov/me-cfs/symptoms-diagnosis/. Accessed 11 Nov 2023
Bonilla H, Quach TC, Tiwari A et al (2023) Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic. Front Neurol 14. https://doi.org/10.3389/fneur.2023.1090747
Buonsenso D, Di GL, De RC et al (2022) Long-term outcomes of pediatric infections: from traditional infectious diseases to long Covid. Future Microbiol 17:551–571. https://doi.org/10.2217/fmb-2022-0031
González-Hermosillo JA, Martínez-López JP, Carrillo-Lampón SA et al (2021) Post-acute COVID-19 symptoms, a potential link with myalgic encephalomyelitis/chronic fatigue syndrome: a 6-month survey in a Mexican cohort. Brain Sci 11:760. https://doi.org/10.3390/brainsci11060760
Vernon SD, Hartle M, Sullivan K et al (2023) Post-exertional malaise among people with long COVID compared to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Work 74:1179–1186. https://doi.org/10.3233/WOR-220581
Deutsche Gesellschaft für ME/CSF e. V. (2023) Post-exertional malaise. https://www.mecfs.de/was-ist-me-cfs/pem/. Accessed 11 Nov 2023
Kedor C, Freitag H, Meyer-Arndt L et al (2022) A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 13:5104. https://doi.org/10.1038/s41467-022-32507-6
Kedor C, Freitag H, Meyer-Arndt L et al (2022) Author Correction: A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 13:6009. https://doi.org/10.1038/s41467-022-33784-x
Peo LC, Wiehler K, Paulick J, Gerrer K, Leone A, Viereck A, Haegele M, Stojanov S, Warlitz C, Augustin S, Alberer M, Hattesohl DBR, Froehlich L, Scheibenbogen C, Pricoco RBU. Pediatric and adult patients with ME/CFS following COVID-19: a structured approach to diagnosis using the Munich Berlin Symptom Questionnaire (MBSQ). In: https://www.medrxiv.org/
Rowe K (2023) Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in adolescents: practical guidance and management challenges. Adolesc Health Med Ther 14:13–26. https://doi.org/10.2147/AHMT.S317314
Rasa S, Nora-Krukle Z, Henning N et al (2018) Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 16:268. https://doi.org/10.1186/s12967-018-1644-y
Bannister BA (1988) Post-infectious disease syndrome. Postgrad Med J 64:559–567. https://doi.org/10.1136/pgmj.64.753.559
Choutka J, Jansari V, Hornig M, Iwasaki A (2022) Author Correction: Unexplained post-acute infection syndromes. Nat Med 28:1723–1723. https://doi.org/10.1038/s41591-022-01952-7
Choutka J, Jansari V, Hornig M, Iwasaki A (2022) Unexplained post-acute infection syndromes. Nat Med 28:911–923. https://doi.org/10.1038/s41591-022-01810-6
Katz BZ, Collin SM, Murphy G et al (2018) The international collaborative on fatigue following infection (COFFI). Fatigue 6:106–121. https://doi.org/10.1080/21641846.2018.1426086
Katz BZ, Shiraishi Y, Mears CJ et al (2009) Chronic fatigue syndrome after infectious mononucleosis in adolescents. Pediatrics 124:189–193. https://doi.org/10.1542/peds.2008-1879
Rowe KS (2019) Long term follow up of young people with chronic fatigue syndrome attending a pediatric outpatient service. Front Pedia 7. https://doi.org/10.3389/fped.2019.00021
Buchhorn R (2022) Dysautonomia in children with post-acute sequelae of coronavirus 2019 disease and/or vaccination. Vaccines 10:1686. https://doi.org/10.3390/vaccines10101686
Scholkmann F, May C-A (2023) COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvac-syndrome”): similarities and differences. Pathology - Research and Practice 246:154497. https://doi.org/10.1016/j.prp.2023.154497
Engels G, Hecker K, Forster J et al (2022) High seroprevalence of SARS-CoV-2 in preschool children in July 2022. Dtsch Arztebl Int. https://doi.org/10.3238/arztebl.m2022.0345
Ott R, Achenbach P, Ewald DA et al (2022) SARS-CoV-2 seroprevalence in preschool and school-age children. Dtsch Arztebl Int. https://doi.org/10.3238/arztebl.m2022.0355
Pricoco R, Meidel P, Hofberger T, Zietemann H, Mueller Y, Wiehler K, Michel K, Paulick J, Leone A, Haegele M, Mayer-Huber S. One-year follow-up of young people with ME/CFS following infectious mononucleosis by Epstein-Barr virus. Posted 27/07/23 on MedRxiv: https://doi.org/10.1101/2023.07.24.23293082v1
Nittas V, Gao M, West EA et al (2022) Long COVID through a public Health lens: an umbrella review. Public Health Rev 43. https://doi.org/10.3389/phrs.2022.1604501
Behnood SA, Shafran R, Bennett SD et al (2022) Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect 84:158–170. https://doi.org/10.1016/j.jinf.2021.11.011
Morello R, Martino L, Buonsenso D (2023) Diagnosis and management of post-COVID (Long COVID) in children: a moving target. Curr Opin Pediatr 35:184–192. https://doi.org/10.1097/MOP.0000000000001221
Mohandas S, Jagannathan P, Henrich TJ et al (2023) Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 12. https://doi.org/10.7554/eLife.86014
Rojas M, Rodríguez Y, Acosta-Ampudia Y et al (2022) Autoimmunity is a hallmark of post-COVID syndrome. J Transl Med 20:129. https://doi.org/10.1186/s12967-022-03328-4
Lammi V, Nakanishi T, Jones SE, Andrews SJ, Karjalainen J, Cortes B, O'Brien HE, Fulton-Howard BE, Haapaniemi HH, Schmidt A, Mitchell RE et al. Genome-wide association study of long COVID. Posted 01/07/23 on medRxiv: https://doi.org/10.1101/2023.06.29.23292056
Szewczykowski C, Mardin C, Lucio M et al (2022) Long COVID: association of functional autoantibodies against G-protein-coupled receptors with an impaired retinal microcirculation. Int J Mol Sci 23:7209. https://doi.org/10.3390/ijms23137209
Buonsenso D, Morello R, Mariani F et al (2023) Risk of long Covid in children infected with Omicron or <scp>pre-Omicron SARS-CoV</scp> -2 variants. Acta Paediatr 112:1284–1286. https://doi.org/10.1111/apa.16764
Antonelli M, Pujol JC, Spector TD et al (2022) Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. The Lancet 399:2263–2264. https://doi.org/10.1016/S0140-6736(22)00941-2
Ayoubkhani D, Bermingham C, Pouwels KB et al (2022) Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ e069676. https://doi.org/10.1136/bmj-2021-069676
Sivan M, Greenhalgh T, Milne R, Delaney B (2022) Are vaccines a potential treatment for long covid? BMJ o988. https://doi.org/10.1136/bmj.o988
Watanabe A, Iwagami M, Yasuhara J et al (2023) Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis. Vaccine 41:1783–1790. https://doi.org/10.1016/j.vaccine.2023.02.008
Selvakumar J, Havdal LB, Drevvatne M et al (2023) Prevalence and characteristics associated with post–COVID-19 condition among nonhospitalized adolescents and young adults. JAMA Netw Open 6:e235763. https://doi.org/10.1001/jamanetworkopen.2023.5763
Lund LC, Hallas J, Nielsen H et al (2021) Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis 21:1373–1382. https://doi.org/10.1016/S1473-3099(21)00211-5
Nugawela MD, Stephenson T, Shafran R et al (2022) Predictive model for long COVID in children 3 months after a SARS-CoV-2 PCR test. BMC Med 20:465. https://doi.org/10.1186/s12916-022-02664-y
U. S. National Library of Medidine ClinicalTrials.gov Genetic risk factors for multi-system inflammatory syndrome in children and pediatric post COVID condition (GRIP). ClinicalTrials.gov Identifier: NCT05722717. https://classic.clinicaltrials.gov/ct2/show/NCT05722717. Accessed 11 Nov 2023
Goretzki SC, Brasseler M, Dogan B et al (2023) High prevalence of alternative diagnoses in children and adolescents with suspected long COVID—a single center cohort study. Viruses 15:579. https://doi.org/10.3390/v15020579
Racine N, McArthur BA, Cooke JE et al (2021) Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19. JAMA Pediatr 175:1142. https://doi.org/10.1001/jamapediatrics.2021.2482
Ravens-Sieberer U, Devine J, Napp A-K et al (2023) Three years into the pandemic: results of the longitudinal German COPSY study on youth mental health and health-related quality of life. Front Public Health 11. https://doi.org/10.3389/fpubh.2023.1129073
Blankenburg J, Wekenborg MK, Reichert J et al (2022) Comparison of mental health outcomes in seropositive and seronegative adolescents during the COVID19 pandemic. Sci Rep 12:2246. https://doi.org/10.1038/s41598-022-06166-y
Kikkenborg Berg S, Dam Nielsen S, Nygaard U et al (2022) Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health 6:240–248. https://doi.org/10.1016/S2352-4642(22)00004-9
Stephenson T, Pinto Pereira SM, Nugawela MD et al (2023) Long COVID—six months of prospective follow-up of changes in symptom profiles of non-hospitalised children and young people after SARS-CoV-2 testing: a national matched cohort study (The CLoCk) study. PLoS ONE 18:e0277704. https://doi.org/10.1371/journal.pone.0277704
Lorman V, Rao S, Jhaveri R et al (2023) Understanding pediatric long COVID using a tree-based scan statistic approach: an EHR-based cohort study from the RECOVER Program. JAMIA Open 6. https://doi.org/10.1093/jamiaopen/ooad016
Calcaterra V, Tagi VM, De Santis R et al (2023) Endocrinological involvement in children and adolescents affected by COVID-19: a narrative review. J Clin Med 12:5248. https://doi.org/10.3390/jcm12165248
Hyatt KH, Jacobson LB, Schneider VS (1975) Comparison of 70 degrees tilt, LBNP, and passive standing as measrues of orthostatic tolerance. Aviat Space Environ Med 46:801–808
Malone LA, Morrow A, Chen Y et al (2022) Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of postacute sequelae of SARS-CoV-2 infection (PASC) in children and adolescents. PM&R 14:1241–1269. https://doi.org/10.1002/pmrj.12890
Buonsenso D, Di Giuda D, Sigfrid L et al (2021) Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc Health 5:677–680. https://doi.org/10.1016/S2352-4642(21)00196-6
Heiss R, Tan L, Schmidt S et al (2023) Pulmonary dysfunction after pediatric COVID-19. Radiology 306. https://doi.org/10.1148/radiol.221250
Cotler J, Holtzman C, Dudun C, Jason L (2018) A brief questionnaire to assess post-exertional malaise. Diagnostics 8:66. https://doi.org/10.3390/diagnostics8030066
Stussman B, Calco B, Norato G, Gavin A, Chigurupati S, Nath AWB. A mixed methods system for the assessment of post exertional malaise in encephalomyelitis/chronic fatigue syndrome. Posted 26/04/23 on medRxiv: https://doi.org/10.1101/2023.04.24.23288821
Töpfner N, Brinkmann F (2023) Long-/post-COVID-19 bei Kindern und Jugendlichen. Monatsschrift Kinderheilkunde 171:601–607. https://doi.org/10.1007/s00112-023-01782-y
Jason LA, Brown M, Brown A et al (2013) Energy conservation/envelope theory interventions. Fatigue 1:27–42. https://doi.org/10.1080/21641846.2012.733602
Goudsmit EM, Nijs J, Jason LA, Wallman KE (2012) Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: a consensus document. Disabil Rehabil 34:1140–1147. https://doi.org/10.3109/09638288.2011.635746
Scheibenbogen C, Bellmann-Strobl JT, Heindrich C et al (2023) Fighting post-COVID and ME/CFS – development of curative therapies. Front Med 10. https://doi.org/10.3389/fmed.2023.1194754
Becker N-P, Haberland A, Wenzel K et al (2020) A three-part, randomised study to investigate the safety, tolerability, pharmacokinetics and mode of action of BC 007, neutraliser of pathogenic autoantibodies against G-protein coupled receptors in healthy, young and elderly subjects. Clin Drug Investig 40:433–447. https://doi.org/10.1007/s40261-020-00903-9
Zilberman-Itskovich S, Catalogna M, Sasson E et al (2022) Hyperbaric oxygen therapy improves neurocognitive functions and symptoms of post-COVID condition: randomized controlled trial. Sci Rep 12:11252. https://doi.org/10.1038/s41598-022-15565-0
U. S. National Library of Medidine ClinicalTrials.gov AT1001 for the treatment of long COVID. ClinicalTrials.gov Identifier: NCT05747534. https://classic.clinicaltrials.gov/ct2/show/NCT05747534. Accessed 11 Nov 2023
U. S. National Library of Medidine ClinicalTrials.gov The roles of vitamin D and microbiome in children with post-acute COVID-19 syndromes (PACS) and long COVID. ClinicalTrials.gov Identifier: NCT05633472. https://classic.clinicaltrials.gov/ct2/show/NCT05633472. Accessed 11 Nov 2023
Borch L, Holm M, Knudsen M et al (2022) Long COVID symptoms and duration in SARS-CoV-2 positive children — a nationwide cohort study. Eur J Pediatr 181:1597–1607. https://doi.org/10.1007/s00431-021-04345-z
Office for National Statistics (ONS) (2023) Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/2february2023
Smith MS, Martin-Herz SP, Womack WM, Marsigan JL (2003) Comparative study of anxiety, depression, somatization, functional disability, and illness attribution in adolescents with chronic fatigue or migraine. Pediatrics 111:e376–e381. https://doi.org/10.1542/peds.111.4.e376
Nijhof SL, Maijer K, Bleijenberg G et al (2011) Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics 127:e1169–e1175. https://doi.org/10.1542/peds.2010-1147
Crawley EM, Emond AM, Sterne JAC (2011) Unidentified chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a major cause of school absence: surveillance outcomes from school-based clinics. BMJ Open 1:e000252–e000252. https://doi.org/10.1136/bmjopen-2011-000252
Crawley E, Sterne JAC (2009) Association between school absence and physical function in paediatric chronic fatigue syndrome/myalgic encephalopathy. Arch Dis Child 94:752–756. https://doi.org/10.1136/adc.2008.143537
Dowsett EG, Colby J (1997) Long-term sickness absence due to ME/CFS in UK schools. J Chronic Fatigue Syndr 3:29–42. https://doi.org/10.1300/J092v03n02_04
El Khoury J, Skoury M, El Khoury MY (2023) The impact of post-COVID-19 syndrome in adolescents: a pilot study. Cureus. https://doi.org/10.7759/cureus.40655
European Commission (EU) (2022) Education and Training Monitor. Country Reports. https://op.europa.eu/webpub/eac/education-and-training-monitor-2022/en/country-reports/country-reports.html
Froehlich L, Hattesohl DBR, Jason LA et al (2021) Medical care situation of people with myalgic encephalomyelitis/chronic fatigue syndrome in Germany. Medicina 57:646. https://doi.org/10.3390/medicina57070646
Froehlich L, Hattesohl DB, Cotler J et al (2022) Causal attributions and perceived stigma for myalgic encephalomyelitis/chronic fatigue syndrome. J Health Psychol 27:2291–2304. https://doi.org/10.1177/13591053211027631
Huckleberry Films Message in a bottle - long Covid SOS. https://www.youtube.com/watch?v=IIeOoS_A4c8. Accessed 11 Nov 2023
The BMJ Opinion (2020) Counting long covid in children. Accessed 11/11/23 via: https://blogs.bmj.com/bmj/2020/10/16/counting-long-covid-in-children/
Au L, Capotescu C, Eyal G, Finestone G (2022) Long covid and medical gaslighting: dismissal, delayed diagnosis, and deferred treatment. SSM - Qualitative Research in Health 2:100167. https://doi.org/10.1016/j.ssmqr.2022.100167
Meyerson WU, Hoyle RH (2023) Pre-pandemic activity on a myalgic encephalomyelitis/chronic fatigue syndrome support forum is highly associated with later activity on a long COVID support forum for a variety of reasons: a mixed methods study. PLoS One Sep 8;18(9):e0291173. https://doi.org/10.1371/journal.pone.0291173
LONG COViD KiDS We believe all children should be able to thrive and look forward to a positive future. https://www.longcovidkids.org/contact-9. Accessed 11 Nov 2023
Elterninitiative ME/CSF-kranke Kinder & Jugendliche München e. V. (2023) Was ist ME/CFS?, ME/CFS und was jetzt? https://www.mecfs-kinder-muc.de/. Accessed 11 Nov 2023
NICHT GENESEN KIDS (2023) Wir suchen alle Eltern betroffener Kinder & Jugendlicher deutschlandweit mit Post Covid, Post Vac, ME/CFS. https://nichtgenesenkids.org/. Accessed 11 Nov 2023
Kokorelis C, Malone L, Byrne K et al (2023) Onset of postural orthostatic tachycardia syndrome (POTS) following COVID-19 infection: a pediatric case report. Clin Pediatr 62:92–95. https://doi.org/10.1177/00099228221113609
Petracek LS, Broussard CA, Swope RL, Rowe PC (2023) A case study of successful application of the principles of ME/CFS care to an individual with long COVID. Healthcare 11:865. https://doi.org/10.3390/healthcare11060865
Petracek LS, Suskauer SJ, Vickers RF et al (2021) Adolescent and young adult ME/CFS after confirmed or probable COVID-19. Front Med 8. https://doi.org/10.3389/fmed.2021.668944
van Campen C (Linda) MC, Rowe PC, Verheugt FWA, Visser FC (2021) Numeric rating scales show prolonged post-exertional symptoms after orthostatic testing of adults with myalgic encephalomyelitis/chronic fatigue syndrome. Front Med 7. https://doi.org/10.3389/fmed.2020.602894
Ocon AJ, Messer ZR, Medow MS, Stewart JM (2012) Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome. Clin Sci 122:227–238. https://doi.org/10.1042/CS20110241
Bou-Holaigah I (1995) The relationship between neurally mediated hypotension and the chronic fatigue syndrome. JAMA 274:961. https://doi.org/10.1001/jama.1995.03530120053041
Eder J, Schumm L, Armann JP et al (2023) Increased red blood cell deformation in children and adolescents after SARS-CoV-2 infection. Sci Rep 13:9823. https://doi.org/10.1038/s41598-023-35692-6
U. S. National Library of Medidine ClinicalTrials.gov Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (ME/CFS) Registry and Biobank, COVID-19, SARS-CoV-2 (MECFS-R). ClinicalTrials.gov Identifier: NCT05778006. https://classic.clinicaltrials.gov/ct2/show/NCT05778006?term=NCT05778006&draw=2&rank=1. Accessed 11 Nov 2023
U. S. National Library of Medidine ClinicalTrials.gov Munich Long COVID Registry Study for Children, Adolescents and Adults (MLC-R) (MLC-R). ClinicalTrials.gov Identifier: NCT05638724. https://classic.clinicaltrials.gov/ct2/show/NCT05638724?term=NCT05638724&draw=2&rank=1. Accessed 11 Nov 2023
Förderinitiative für die Versorgungsforschung zum Post-COVID-Syndrom Teilprojekt 2. Post-COVID Kids Bavaria – PCFC (Post-COVID Fatigue Center). https://www.lgl.bayern.de/downloads/gesundheit/infektionsschutz/doc/post_covid_kids_bavaria_teilprojekt_2.pdf. Accessed 11 Nov 2023
Charité Fatigue Centrum Das Fatigue Centrum der Charité — Universitätsmedizin Berlin. https://cfc.charite.de/forschung/immme/team/. Accessed 11 Nov 2023
NINDS Common Data Elements Myalgic encephalomyelitis/chronic fatigue syndrome. https://www.commondataelements.ninds.nih.gov/MyalgicEncephalomyelitis/ChronicFatigueSyndrome. Accessed 11 Nov 2023
Munblit D, Nicholson T, Akrami A et al (2022) A core outcome set for post-COVID-19 condition in adults for use in clinical practice and research: an international Delphi consensus study. Lancet Respir Med 10:715–724. https://doi.org/10.1016/S2213-2600(22)00169-2
Munblit D, Buonsenso D, Sigfrid L et al (2022) Post-COVID-19 condition in children: a COS is urgently needed. Lancet Respir Med 10:628–629. https://doi.org/10.1016/S2213-2600(22)00211-9
Acknowledgements
The authors thank the patients, legal guardians, parents, patient advocacy groups, colleagues, and politicians for fruitful collaboration and support in dealing with this challenging medical condition.
Funding
Open Access funding enabled and organized by Projekt DEAL. Funds, grants, and supports are outlined below. NT is steering committee member of the German Society for Pediatric Infectious diseases. She received research grants for SARS-CoV-2 from the German Federal Ministry of Education and Research as part of the coverCHILD project of the Network University Medicine (NUM) (FKZ 01KX2121). UB, FB, and NT were coauthors of German guidelines for Long-/Post-COVID syndrome as well as organizers and together with SS coauthors of the German consensus statement regarding Long-/Post-COVID syndrome in children and adolescents signed by 19 German Pediatric Societies. UB is a member of Corona Task Force of the German Associations of Pediatrics (DGKJ), the Scientific Advisory Board “Post-COVID-Syndrome” of the Federal Medical Association (BÄK), of the Medical Association Long COVID, the Medical Advisory Board of the German Association for ME/CFS (DG ME/CFS), and the “Elterninitiative ME/CFS-kranke Kinder & Jugendliche e.V.”. She received research grants for PCC and/or ME/CFS from the Federal Ministry of Education and Research (BMBF), the Federal Ministry of Health (BMG), the Bavarian Ministry of Health and Care (StMGP), the Bavarian Ministry of Science and Arts (StMWK), the German Center for Infection Research (DZIF), the Menschen für Kinder-Foundation, the Weidenhammer-Zoebele Foundation, the Lost-Voices Foundation, and the ME/CFS Research Foundation, as well as travel grants or lecture fees from the Weidenhammer-Zoebele Foundation, the Bavarian Association of Pharmacists (BLAK), the German Association of Pediatric Rehabilitation, and the ME/CFS Research Foundation.
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toepfner, N., Brinkmann, F., Augustin, S. et al. Long COVID in pediatrics—epidemiology, diagnosis, and management. Eur J Pediatr 183, 1543–1553 (2024). https://doi.org/10.1007/s00431-023-05360-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05360-y