Abstract
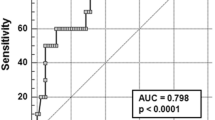
Magnetic resonance imaging (MRI) T2* is the gold standard for detecting iron deposition in cardiac tissue, but the technique has limitations and cannot be fully performed in paediatric thalassemia patients. The aim of this study was to analyse clinical data to identify other predictors of cardiac iron deposition. A retrospective analysis was performed on 370 children with β-TM. According to the cardiac MRI results, patients were allocated to a cardiac deposition group and noncardiac deposition group. Multivariate analysis revealed that genotype and corrected QT interval were associated with cardiac iron deposition, indicating that the-β0/β0 genotype conferred greater susceptibility to cardiac iron deposition. Receiver operating characteristic curve (ROC) analysis was performed, and the area under the curve (AUC) of genotype was 0.651. The AUC for the corrected QT interval was 0.711, at a cut-off value of 418.5 ms. ROC analysis of the combined genotype and corrected QT interval showed an AUC of 0.762 with 81.3% sensitivity and 64.7% specificity. Compared to patients with the β+/β+ and β0β+ genotypes, β0β0 children with β-TM were more likely to have cardiac iron deposition.
Conclusion: The genotype and QTc interval can be used to predict cardiac iron deposition in children with β-TM who are unable to undergo MRI T2 testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
β-TM is a chronic haemolytic anaemia. High power circulation increases cardiac deposition. Endothelial dysfunction, intimal remodelling and peroxidative damage due to cellular hypoxia and iron overload can lead to myocardial ischaemia and tissue fibrosis, resulting in cardiac injury [1, 2]. Even in paediatric β-TM patients, manifestations of cardiac damage, such as LV dilatation and LV diastolic dysfunction can occur [3]. Cardiac iron deposition is a major cause of death in β-thalassemia major (β-TM). Early diagnosis and treatment can reverse and improve patient survival.
MRI T2* is a noninvasive and highly sensitive test that is the gold standard for the early detection of iron deposits in the cardiac tissue [4,5,6,7,8,9]. Cardiac MRI T2* can detect and quantify the iron deposition in the cardiac. Survival rates of transfusion-dependent TM patients have improved significantly since the use of MRI T2* techniques [11]. However, this technique requires high levels of physicians and technologists, expensive instruments and equipment and examinations, cannot be carried out in some hospitals, and is challenging for preschool children in terms of cooperation; so MRI T2* cannot be fully implemented in paediatric TM patients.
Therefore, it is crucial to find common clinical indicators that can be used to predict iron deposition in the heart.
Methods
We retrospectively analysed the clinical data collected from patients with β-TM at the Department of Paediatrics, the First Affiliated Hospital of Guangxi Medical University, between January 2017 and October 2022. The following inclusion criteria were employed: (1) age range 3 to 14 years and (2) cardiac MRI T2* and genetic testing for TM. The following exclusion criteria were employed: (1) patients with infection, (2) severe malnutrition, (3) TM in combination with other inherited haemoglobin disorders and (4) TM in combination with diabetes, chromosomal disorders or malignancy.
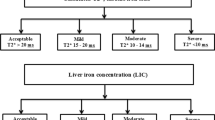
General data were collected, including sex, age, height, weight, body mass index (BMI), thalassaemia genotypes, hydroxyurea and chelation therapy. First, as our study was a retrospective analysis, blood samples, MRI and genotype data were obtained from hospital laboratories. The blood samples were peripheral blood from the children. MRI method: the pretransplant magnetic resonance imaging (MRI) of all patients was performed using a 3.0 T scanner (Verio, Siemens, Germany), and data were analysed using CMR Tools software (England). Genetic testing for thalassemia was performed in a hospital laboratory. β+ abnormal genes are IVS -II- 654, - 28, βE, - 29, - 30, - 32, CAP and IVS -I- 5. β0 abnormal genes are CD41-42, CD17, CD71-72, Int, CD31, CD14-15, IVS -I- 1, CD43, CD27-28, CD14-15, IVS -I- 1, CD43 and CD27-28. Clinical variables were defined as follows: Hepatomegaly > 2 cm below the right costal margin and splenomegaly > 2 cm below the left costal margin. Cardiac MRI T2* results were recorded. Cardiac T2* is a quantitative measurement of tissue iron content that correlates negatively with tissue iron content and can be used to diagnose the presence of iron deposition in the myocardium. According to the results of cardiac MRI T2* (normal (T2* > 20 ms), mild (14 ms ≤ T2* < 20 ms), moderate (10 ms ≤ T2* < 14 ms) and severe (T2* < 10 ms) [10]), patients were categorized into a cardiac deposition group and a normal group (noncardiac deposition). Laboratory data were collected within 3 months before or after MRI detection. Laboratory indices included routine blood count, liver and kidney function, cardiac enzymes and echocardiography and abdominal ultrasound. Specifically, routine blood indicators included white blood cell (WBC) count, red blood cell count, platelet count, haemoglobin concentration, mean cellular volume(MCV), mean cellular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and red blood cell distribution width (RDW); liver function indicators included total bilirubin, direct bilirubin, indirect bilirubin, total bile acids, alanine aminotransferase (ALT) and aspartate aminotransferase (AST); indicators of cardiac enzymes included creatine kinase (CK), creatine kinase isoenzyme MB (CK-MB) and lactate dehydrogenase; ECG indicators included heart rate, PR interval duration, QRS interval duration, QT interval duration and corrected QT interval; cardiac ultrasound indicators include left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD), right ventricular internal diameter (RVID), left ventricular (LV) fractional shortening, LV ejection fraction, stroke volume, cardiac output and LV end-diastolic volume.
Data were analysed using SPSS 26 software. Data with a normal distribution were expressed as the mean ± standard deviation. Two-group comparisons were performed using the independent t test. Data that did not have a normal distribution were expressed as the median (four-digit interval) [P50(P25, P75)]. Two-group comparisons were performed using the Mann–Whitney U test. Proportion data are presented as numbers (percentages). Pearson’s Chi-square test was used to perform multiple group comparisons. To minimize the effect of age on some of the cardiac ultrasound parameters, an analysis of covariance (ANCOVA) was performed with age as the covariate and the measured parameters as the dependent variable. Correlations between variables were analysed by Spearman’s test. Significant indices were analysed using multivariate logistic regression analysis to determine risk factors. The optimum threshold for the significant parameter was constructed using receiver operating characteristic (ROC) curves. Two-sided P values < 0.05 were considered significant for all analyses.
Results
Over the period of observation, a total of 470 patients with β-TM who underwent cardiac MRI T2* were enrolled. One hundred patients were excluded because of incomplete data. Of the 370 patients included, 64 (17.30%) had cardiac iron deposition, and 306 (82.7%) had no cardiac iron deposition. Sixty-two of 64 patients with cardiac iron deposition and 266 of 306 patients with noncardiac iron deposition had combined liver iron deposition. All patients were treated with regular transfusion and iron chelation. The number of cases treated with deferoxamine alone or the combination of deferoxamine and deferasirox was small. Therefore, these two cases of chelation therapy were included in the deferasirox combined with deferoxamine group.
The distribution of age and genotypes differed statistically between the two groups (p < 0.001). The differences among the three genotypes were mainly as follows: the incidence of cardiac iron deposition was higher with the β0/β0 genotype than with the β0/β+ (p < 0.001) and β+ /β+ (p = 0.011) genotypes. There was no statistically significant difference between the incidence of cardiac iron deposition in patients with the β0/β+ and β+ /β+ genotypes (p = 0.714). There were no significant differences in sex, BMI, chelation therapy, hydroxyurea therapy, hepatomegaly or splenomegaly between the two groups (p > 0.05). The clinical characteristics of the patients are shown in Table 1.
In terms of laboratory indicators, indirect bilirubin, total bile acids, ALT, AST, CK, CK-MB, lactate dehydrogenase, PR interval, QT interval, QTc interval, LVEDD, LVESD, RVID, stroke volume, cardiac output and LV end-diastolic volume between the two groups were statistically significant. (all, p < 0.05). There were no significant differences in other indicators.
The cardiac ultrasound indices LVEDD, LVESD, RVID, stroke volume, cardiac output and LV end-diastolic volume were affected by age. Age was approximately normally distributed in the cardiac iron deposition group and normally distributed in the noncardiac iron deposition group. The age, LVEDD, LVESD, RVID, stroke volume, cardiac output and LV end-diastolic volume of the samples in each group had uniform variance, and the correlation coefficients of the six cardiac ultrasound indices with age were the same, which satisfied the conditions of analysis of covariance. The differences in LVEDD, LVESD, RVID, stroke volume, cardiac output and LV end-diastolic volume between the two groups after correction were not statistically significant (all, p > 0.05), as shown in Table 2.
Statistically different indicators and cardiac MRI T2* values were analysed by correlation, indicators that did not have a linear relationship with cardiac MRI T2* values were excluded, and the indicators entered into the multifactorial analysis had a low degree of correlation (correlation coefficient < 0.5) or were not correlated (Supplementary File 1). Final inclusion of age, genotype, indirect bilirubin, AST, CK-MB, lactate dehydrogenase and corrected QT interval row logistic analysis. Multivariate logistic regression analysis showed that genotype and a high corrected QT interval were associated with cardiac iron deposition, as shown in Table 3.
Genotype, corrected QT interval and combination of two variables were analysed by ROC curves, and ROC curve results are shown in Fig. 1 and Table 4.
Discussion
β-TM is a classic secondary iron overload disease. Excessive iron deposition in the heart leads to dilated cardiomyopathy and cardiac failure, which are the main causes of death in patients [12,13,14,15]. Early identification and treatment of cardiac iron deposition may improve patient prognosis and increase survival. Defects in the β-globin gene, resulting in the absence (β0) or reduction (β+) of β-globin chain synthesis, are characteristic of β-TM. The degree of clinical disease varies among β-TM patients with different genotypes. Patients with major TM require regular blood transfusions for a long time after the onset of the disease, and the older they are and the more frequent the transfusions are, the greater the probability of organ iron deposition occurring. In the present study, we found that the age of patients in the cardiac iron deposition group was higher than that of patients in the noncardiac iron deposition group; in addition, there was a correlation between age and cardiac iron deposition, but increasing age was not a factor that promoted the occurrence of cardiac iron deposition. Differences in genotype can also exhibit varying degrees of cardiac iron deposition. Sagar et al. found that iron toxicity-induced DNA damage was greater in β0 homozygotes than in β0 heterozygotes or β0β+ heterozygotes [16]. Pistoia et al. conducted a cross-sectional study of MRI T2* values and cardiac iron deposition in different genomes of β-TM patients and found that β0 homozygotes and β0β+ heterozygous patients had higher levels of cardiac iron deposition than β+ homozygotes [17]. Another study showed that β0 homozygotes and β0β+ patients were at higher risk of cardiac iron deposition and LV dysfunction [18]. Our study found a higher incidence of cardiac iron deposition with the β0/β0 genotype than with the β0/β+ and β+ /β + genotypes, which is consistent with the above findings. We also found that the β0/β0 genotype promotes the development of iron deposition in the cardiac tissue. However, since β0/β0 patients have a clinical presentation of TM major with an early onset and a high number of transfusions and are prone to iron overload, the effect of the number and volume of transfusions on this conclusion cannot be excluded.
Excessive iron deposition in the liver can lead to liver damage, liver fibrosis, cirrhosis and even hepatocellular carcinoma [19]. Although ALT and AST are mainly used to evaluate liver impairment, ALT and AST play a considerable role in the development of cardiovascular disease [20,21,22] and are also associated with endothelial dysfunction and coronary artery disease [23]. In the present study, we found that the levels of ALT and AST were higher in the cardiac iron deposition group than in the noncardiac iron deposition group, and there was a negative correlation between ALT and cardiac iron deposition (Supplementary Material 1). However, 62 of the 64 children with cardiac iron deposition in this study also had liver iron deposition, so the effect of liver iron deposition on transaminases cannot be excluded. Therefore, whether elevated transaminases in this study can be used to evaluate iron deposition in the cardiac tissue of children with TM needs further study.
Myocardial enzyme profile indicators such as creatine kinase, creatine kinase isoenzyme and LDH are commonly used in clinical practice to assess myocardial damage. The myocardial enzyme profile tends to increase as the degree of myocardial damage increases. However, few studies have focused on the effects of cardiac iron deposition on myocardial enzyme profiles. In this study, the indices of the myocardial enzyme profile did not increase significantly due to the presence of cardiac iron deposition but were lower than those in the noncardiac iron deposition group, indicating that the presence of cardiac iron deposition in β-TM patients cannot be determined by myocardial enzyme-related indices. Moreover, the cardiac enzyme profile is still affected by multiple factors, such as impaired liver function, skeletal muscle damage and tumours [24,25,26]. The higher cardiac enzymes in the noncardiac iron deposition group than in the cardiac iron deposition group in this study may be related to the combination of severe liver iron deposition in some cases in the noncardiac iron deposition group.
Common electrophysiological abnormalities in patients with cardiac iron deposition include sinus bradycardia, prolonged PR intervals, prolonged QRS duration, elevated or reduced QRS voltage, elevated or reduced ST segment and premature ventricular and atrial beats [27]. The effect of iron deposition on the electrical activity of the cardiac system can cause arrhythmias in TM patients [28]. Patsourakos et al. found a higher incidence of prolonged PR interval, atrial fibrillation and late potentials in β-TM patients than in healthy adults [29]. In the present data, we found longer PR interval in the cardiac iron deposition group than in the noncardiac iron deposition group, which is the same as the results of the above study. However, there was no linear correlation between PR intervals and cardiac MRI T2* values in the 370 patients in this study. However, prolonged PR intervals in β-TM patients suggest the presence of atrioventricular conduction disturbances. Although first-degree atrial ventricular block by itself does not pose a life-threatening condition, patients with β-TM should have long-term ECG monitoring for early recognition of atrial fibrillation and early treatment [30].
TM patients have a high incidence of prolonged QT interval and sudden cardiogenic death [31,32,33]. Several studies have shown that prolonged QT interval and QTc interval in β-TM patients are associated with severe cardiac iron deposition [34,35,36], which is seen to cause prolonged QT interval and QTc interval in β-TM patients. In the present study, the QT interval and QTc interval were significantly longer in the children with cardiac iron deposition than in the noncardiac iron deposition group, and there was a correlation between the QTc interval and cardiac iron deposition, which is consistent with the findings of Aggarwal et al., who found that prolonged QRS duration, QT interval and QTc interval in β-TM patients were associated with decreased cardiac MRI T2* values [37]. This study also found that a prolonged QTc interval could be used to predict cardiac iron deposition with a cut-off value of 418.5 ms. However, because the QTc interval showed a low correlation with cardiac iron deposition in this study, and the AUC area was low, the specificity was only 37.9%. Therefore, whether the QTc interval can be used to predict cardiac iron deposition in β-TM patients remains to be studied in large samples and multicentre studies.
In iron deposition cardiomyopathy, LV diastolic dysfunction usually precedes systolic dysfunction [38, 39]. The early stages of cardiac iron deposition lead to diastolic dysfunction and subsequently restrictive cardiomyopathy. Without early recognition and appropriate iron chelation therapy, the disease can eventually progress to end-stage dilated cardiomyopathy. Cardiac MRI T2* values are the most effective tool to accurately assess myocardial iron overload and help guide iron chelation therapy [9].
Cardiac ultrasound combined with cardiac MRI T2* values are sensitive and specific for the early detection of myocardial function and cardiac iron deposition [40, 41]. However, developing countries and low-income countries lack the appropriate equipment. Therefore, cardiac ultrasound is often used to assess early myocardial dysfunction in paediatric and adult patients with TM [42,43,44]. In this study, we found that in children with thalassemia major, cardiac ultrasound indices did not change significantly in children with cardiac iron deposition, so cardiac ultrasound may not be useful for the early monitoring of cardiac iron deposition.
In conclusion, by studying the clinical data of children with β-TM, we found differences in age, genotype, liver function, myocardial function, electrocardiogram and cardiac ultrasound between children with cardiac iron deposition and those without cardiac iron deposition. We also found that children with genotype β0/β0 β-TM were more likely to develop cardiac iron deposition than children with genotypes β + /β+ and β0/β+ . It was also found that the PR interval and QTc interval were prolonged in children with cardiac iron deposition compared to those with noncardiac iron deposition. Children with β-TM should have early and long-term ECG monitoring for the early identification and treatment of arrhythmias.
We found that the combined β0/β0 genotype and QTc interval could be used to predict cardiac iron deposition in children with β-TM with a sensitivity of 81.3%. The MRI T2* technique remains the gold standard for detecting iron deposition in the cardiac tissue [45]. We believe that genotype and QTc interval can be used to identify patients at risk for cardiac iron deposition in hospitals without this technology or in children who cannot cooperate. However, it is still necessary to follow up the ECG results periodically and to confirm the diagnosis with MRI when conditions permit.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Data are available upon reasonable request.
Change history
30 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00431-023-05357-7
References
Hirsch RE, Sibmooh N, Fucharoen S, Friedman JM (2017) HbE/beta-thalassemia and oxidative stress: the key to pathophysiological mechanisms and novel therapeutics. Antioxid Redox Signal 26:794–813
Fibach E, Dana M (2019) Oxidative stress in beta-thalassemia. Mol Diagn Ther 23:245–261
Mah K, Bruce A, Zahari N, Venner MA, Chow K, Thompson RB, Khoo NS, Tham EB (2020) Tilt-table echocardiography unmasks early diastolic dysfunction in patients with hemoglobinopathies. J Pediatr Hematol Oncol 42:391
Wood JC (2011) Impact of iron assessment by MRI. Hematology Am Soc Hematol Educ Program 2011:443–450
Wood JC (2014) Use of magnetic resonance imaging to monitor iron overload. Hematol Oncol Clin North Am 28:747–764
Wood JC (2015) Estimating tissue iron burden: current status and future prospects. Br J Haematol 170:15–28
Carpenter J-P, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, Sheppard MN, Porter JB, Malcolm Walker J, Wood JC et al (2011) On T2* magnetic resonance and cardiac iron. Circulation 123:1519–1528
Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, Wu D, Taylor J, Westwood MA, Anderson LJ et al (2009) Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 120:1961–1968
Chaosuwannakit N, Makarawate P, Wanitpongpun C (2021) The importance of cardiac T2* magnetic resonance imaging for monitoring cardiac siderosis in thalassemia major patients. Tomography 7:130–138
Kondur AK, Li T, Vaitkevicius P, Afonso L (2009) Quantification of myocardial iron overload by cardiovascular magnetic resonance imaging T2* and review of the literature. Clin Cardiol 32:55–59
Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ (2008) Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson 10:42
Borgna-Pignatti C, Rugolotto S, De Stefano P et al (1998) Survival and disease complications in thalassemia major. Ann N Y Acad Sci 850:227–231. https://doi.org/10.1111/j.1749-6632.1998.tb10479.x
Ladis V, Chouliaras G, Berdousi H et al (2005) Longitudinal study of survival and causes of death in patients with thalassemia major in Greece. Ann N Y Acad Sci 1054:445–450. https://doi.org/10.1196/annals.1345.067
Zurlo MG, De Stefano P, Borgna-Pignatti C et al (1989) Survival and causes of death in thalassaemia major. Lancet 2:27–30. https://doi.org/10.1016/s0140-6736(89)90264-x
Borgna-Pignatti C, Cappellini MD, De Stefano P et al (2005) Survival and complications in thalassemia. Ann N Y Acad Sci 1054:40–47. https://doi.org/10.1196/annals.1345.006
Sagar CS, Kumar R, Sharma DC, Kishor P (2015) DNA damage: beta zero versus beta plus thalassemia. Ann Hum Biol 42(6):585–588
Pistoia L, Meloni A, Salvadori S, Spasiano A, Lisi R, Rosso R, Maggio A, D’Ascola DG, Cuccia L, Mangione M et al (2019) Cardiac involvement by CMR in different genotypic groups of thalassemia major patients. Blood Cells Mol Dis 77:1–7
Pistoia L, Meloni A, Ricchi P, Filosa A, Lisi R, Maggio A, Rosso R, Messina G, Dello Iacono N, Cuccia L et al (2021) Genotypic groups as risk factors for cardiac magnetic resonance abnormalities and complications in thalassemia major: a large, multicentre study. Blood Transfus 19:168–176
Chen J, Li X, Ge C, Min J, Wang F (2022) The multifaceted role of ferroptosis in liver disease. Cell Death Differ 29:467–480
Svein Ivar Bekkelund (2021) Serum alanine aminotransferase activity and risk factors for cardiovascular disease in a Caucasian population: the Tromso study. BMC Cardiovasc Disord 21:29
Yokoyama M, Watanabe T, Otaki Y, Takahashi H, Arimoto T, Shishido T, Miyamoto T, Konta T, Shibata Y, Daimon M et al (2016) Association of the aspartate aminotransferase to alanine aminotransferase ratio with BNP level and cardiovascular mortality in the general population: the Yamagata study 10-year follow-up. Dis Markers 2016:4857917
Mahady SE, Wong G, Turner RM, Mitchell P, Macaskill P, Craig JC, George J (2017) Elevated liver enzymes and mortality in older individuals: a prospective cohort study. J Clin Gastroenterol 51:439–445
Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CDA, Heine RJ, Diamant M (2007) Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis 191:391–396
Samsky MD, Patel CB, DeWald TA et al (2013) Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 61:2397–2405. https://doi.org/10.1016/j.jacc.2013.03.042
Wens SCA, Schaaf GJ, Michels M et al (2016) Elevated plasma cardiac troponin T levels caused by skeletal muscle damage in Pompe disease. Circ Cardiovasc Genet 9:6–13. https://doi.org/10.1161/CIRCGENETICS.115.001322
Reina-Couto M, Pereira-Terra P, Quelhas-Santos J et al (2021) Inflammation in human heart failure: major mediators and therapeutic targets. Front Physiol 12:746494. https://doi.org/10.3389/fphys.2021.746494
Asadian S, Rezaeian N, Hosseini L et al (2021) How does iron deposition modify the myocardium? A feature-tracking cardiac magnetic resonance study. Int J Cardiovasc Imaging 37:3269–3277. https://doi.org/10.1007/s10554-021-02305-0
Walker JM (2013) Thalassaemia major and the heart: a toxic cardiomyopathy tamed? Heart 99:827–34
Patsourakos D, Gatzoulis KA, Aggeli C, Delicou S, Dimitroglou Y, Xydaki K, Toutouzas K, Androulakis A, Tousoulis D (2020) Twelve-lead and signal-averaged electrocardiographic parameters among beta-thalassemia major patients. J Arrhythm 36:920–928
Russo V, Papa AA, Rago A, Nigro G (2016) The importance of a correct methodological approach for the arrhythmic risk evaluation in beta thalassemia major patients. Int J Cardiol 225:107–108
Garadah TS, Kassab S, Mahdi N, Abu-Taleb A, Jamsheer A (2010) QTc interval and QT dispersion in patients with thalassemia major: electrocardiographic (EKG) and echocardiographic evaluation. Clin Med Insights Cardiol 4:31–37
Oztarhan K, Delibas Y, Salcioglu Z, Kaya G, Bakari S, Bornaun H, Aydogan G (2012) Assessment of cardiac parameters in evaluation of cardiac functions in patients with thalassemia major. Pediatr Hematol Oncol 29(3):220–234
Faruqi A, Ahmad SI, Ahmed ST (2016) Evaluation of QT parameters in patients of thalassaemia major with iron overload. J Pak Med Assoc 66:799–802
Kayrak M, Acar K, Gul EE, Ozbek O, Abdulhalikov T, Sonmez O, Alibaşiç H (2012) The association between myocardial iron load and ventricular repolarization parameters in asymptomatic beta-thalassemia patients. Adv Hematol 2012:170510
Detterich J, Noetzli L, Dorey F, Bar-Cohen Y, Harmatz P, Coates T, Wood J (2012) Electrocardiographic consequences of cardiac iron overload in thalassemia major. Am J Hematol 87:139–144
Russo V, Rago A, Pannone B (2011) Dispersion of repolarization and beta-thalassemia major: the prognostic role of QT and JT dispersion for identifying the high-risk patients for sudden death [J. Eur J Haematol 86:324–331
Russo V, Rago A, Pannone B, Papa AA, Di Meo F, Mayer MC, Spasiano A, Russo MG, Golino P, Calabrò R et al (2020) Relation between cardiac T2* values and electrocardiographic parameters in children with transfusion-dependent thalassemia. J Pediatr Hematol Oncol 42:610–614
Gharzuddine WS, Kazma HK, Nuwayhid IA, Bitar FF, Koussa SF, Moukarbel GV, Taher AT (2002) Doppler characterization of left ventricular diastolic function in beta-thalassaemia major. Evidence for an early stage of impaired relaxation. Eur J Echocardiogr 3:47–51
Parale GP, Pawar SS, Tapare VS (2009) Assessment of LV diastolic function in patients with beta-thalassemia major with special reference to E/Eann ratio. J Pediatr Hematol Oncol 31:69–73
Vogel M, Anderson LJ, Holden S, Deanfield JE, Pennell DJ, Walker JM (2003) Tissue Doppler echocardiography in patients with thalassaemia detects early myocardial dysfunction related to myocardial iron overload. Eur Heart J 24:113–119
Aypar E, Alehan D, Hazirolan T, Gümrük F (2010) The efficacy of tissue Doppler imaging in predicting myocardial iron load in patients with beta-thalassemia major: correlation with T2* cardiovascular magnetic resonance. Int J Cardiovasc Imaging 26:413–421
Balci YI, Gurses D (2011) Detection of early cardiac dysfunction in patients with beta-thalassemia major and thalassemia trait by tissue doppler echocardiography. Pediatr Hematol Oncol 28(6):486–496
Kostopoulou AG, Tsiapras DP, Chaidaroglou AS, De Giannis DE, Farmakis D, Kremastinos DT (2014) The pathophysiological relationship and clinical significance of left atrial function and left ventricular diastolic dysfunction in beta-thalassemia major. Am J Hematol 89:13–18
Agha HM, Beshlawy A, Hamdy M, Sobeih A, El Zahrae F, Satar IAAE, AbdelMassih A, Said F, Aziz OAE, El Tagui M et al (2015) Early detection of right ventricular diastolic dysfunction by pulsed tissue Doppler echocardiography in iron loaded beta thalassemia patients. Pediatr Cardiol 36(3):468–474
Abtahi F, Abdi A, Jamshidi S et al (2019) Global longitudinal strain as an indicator of cardiac Iron overload in thalassemia patients. Cardiovasc Ultrasound 17:24. https://doi.org/10.1186/s12947-019-0174-y
Acknowledgements
We thank all the clinicians participating in this study.
Funding
This study was supported by grants from the National Key R&D Program of China (Grant number 2018YFA0507801).
Author information
Authors and Affiliations
Contributions
Yuhang Zhou and Yaxuan Cao designed the study and wrote the manuscript. Yuhang Zhou and Yaxuan Cao analyzed statistics. Yuhang Zhou and Yaxuan Cao contributed equally to the article as co-first authors. Yuhang Zhou, Yaxuan Cao, Zhenhua Fang, Ken Hang, Mengxing Yang, Guanxiu Pang, Jie Zhao, Yang Liu collected and organized the data. Jianming Luo reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and informed consent
According to the Declaration of Helsinki and CIOMS Guidelines, the studies involving human participants were reviewed and approved by Institutional Review Board of First Affiliated Hospital of Guangxi Medical University [2022-KY-(068)]. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s) and minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The co-author name Mengxin Yang has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., Cao, Y., Fang, Z. et al. Research on the clinical factors of cardiac iron deposition in children with beta-thalassemia major. Eur J Pediatr 183, 875–882 (2024). https://doi.org/10.1007/s00431-023-05300-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05300-w