Abstract
This study aims to inform future genetic reanalysis management by evaluating the yield of whole-exome sequencing (WES) reanalysis in standard patient care in the Netherlands. Single-center data of 159 patients with a neurodevelopmental disorder (NDD), in which WES analysis and reanalysis were performed between January 1, 2014, and December 31, 2021, was retrospectively collected. Patients were included if they were under the age of 18 years at initial analysis and if this initial analysis did not result in a diagnosis. Demographic, phenotypic, and genotypic characteristics of patients were collected and analyzed. The primary outcomes of our study were (i) diagnostic yield at reanalysis, (ii) reasons for detecting a new possibly causal variant at reanalysis, (iii) unsolicited findings, and (iv) factors associated with positive result of reanalysis. In addition, we conducted a questionnaire study amongst the 7 genetic department in the Netherlands creating an overview of used techniques, yield, and organization of WES reanalysis. The single-center data show that in most cases, WES reanalysis was initiated by the clinical geneticist (65%) or treating physician (30%). The mean time between initial WES analysis and reanalysis was 3.7 years. A new (likely) pathogenic variant or VUS with a clear link to the phenotype was found in 20 initially negative cases, resulting in a diagnostic yield of 12.6%. In 75% of these patients, the diagnosis had clinical consequences, as for example, a screening plan for associated signs and symptoms could be devised. Most (32%) of the (likely) causal variants identified at WES reanalysis were discovered due to a newly described gene-disease association. In addition to the 12.6% diagnostic yield based on new diagnoses, reclassification of a variant of uncertain significance found at initial analysis led to a definite diagnosis in three patients. Diagnostic yield was higher in patients with dysmorphic features compared to patients without clear dysmorphic features (yield 27% vs. 6%; p = 0.001).
Conclusions: Our results show that WES reanalysis in patients with NDD in standard patient care leads to a substantial increase in genetic diagnoses. In the majority of newly diagnosed patients, the diagnosis had clinical consequences. Knowledge about the clinical impact of WES reanalysis, clinical characteristics associated with higher yield, and the yield per year after a negative WES in larger clinical cohorts is warranted to inform guidelines for genetic reanalysis. These guidelines will be of great value for pediatricians, pediatric rehabilitation specialists, and pediatric neurologists in daily care of patients with NDD.
What is Known: • Whole exome sequencing can cost-effectively identify a genetic cause of intellectual disability in about 30–40% of patients. • WES reanalysis in a research setting can lead to a definitive diagnosis in 10–20% of previously exome negative cases. | |
What is New: • WES reanalysis in standard patient care resulted in a diagnostic yield of 13% in previously exome negative children with NDD. • The presence of dysmorphic features is associated with an increased diagnostic yield of WES reanalysis. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Western countries, intellectual disability (ID), with a global prevalence of 1–8% [1,2,3], is one of the principal socio-economic healthcare problems [3] and is among the conditions with the highest healthcare costs [4]. In Europe, the prevalence of the more broadly defined neurodevelopmental disorders (NDD) is estimated to be around 5–10% [5, 6]. Pathogenic genetic variants are estimated to cause up to 40% of the cases with NDD [7, 8]. Finding a cause for NDD is of great importance to both the patient and the family, providing insight into the prognosis and recurrence risks as well as possible treatment options for some cases [9]. Whole-exome sequencing (WES) is the currently most used technique to screen for pathogenic genetic variants [10]. Knowledge about the clinical impact of WES reanalysis and clinical characteristics associated with higher yield and yield per year after a negative WES in larger clinical cohorts is warranted to inform guidelines for genetic reanalysis. These guidelines will be of great value for pediatricians, pediatric rehabilitation specialists, and pediatric neurologists in daily care of patients with NDD.
Although many diagnoses are made using WES, with a diagnostic yield of around 28% in specific ID cohorts and 36% in cohorts of children with neurodevelopmental delay, many patients remain undiagnosed [11, 12]. Possible explanations are missing the causative variant in the regular exome sequencing pipeline (intronic variants, low coverage, filtering/quality issues) or detection of a variant in a gene not (yet) associated with disease [13, 14]. As variant detection techniques are constantly improving and each year 250 new gene-disease interactions and 9200 new variant-disease associations are described in literature [9], repeating exome analysis after some time can increase diagnostic yield [9, 15, 16].
The yield of WES reanalysis has been studied in multiple research cohorts with varying phenotypes, resulting in yields ranging between 6 and 47% [9, 13, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. A recent systematic review in patients with suspected Mendelian disorders showed an overall diagnostic yield of WES reanalysis of 10% (95% CI 6–13%) [32]. In cohorts with mostly NDD patients, the observed diagnostic yield of reanalysis is between 11 and 18% in larger studies (50 or more reanalyzed cases) and 29% and 36% in two small studies of 14 patients each [9, 13, 17, 20, 22, 29]. Although these studies in research populations indicate that systematic reanalysis of data can improve the diagnostic yield in patients with NDD, these studies do not provide sufficient insight in the benefits of WES reanalysis in standard patient care. A study in clinical patient care, consisting for more than half of patients with NDD, in 2017 showed that WES reanalysis of single patient data after 8–17 months yielded no new diagnoses [33].
Since regular WES reanalysis in all undiagnosed patients with NDD is expected to be associated with high healthcare costs, more information is required on characteristics associated with a high(er) yield. In a research setting, (cost-)effectiveness rises with the increase of interval between analyses [34] and selecting patient groups with a higher chance of a positive result [35].
To gain insight into these parameters as well as the yield of WES reanalysis in standard patient care, we studied a cohort of children with NDD at the Leiden University Medical Centre (LUMC).
Methods
Data collection
We collected data of children with NDD in whom WES analysis and reanalysis were performed between January 1, 2014, and December 31, 2021, in standard patient care in the LUMC. Patients were eligible for reanalysis if initial analysis had not resulted in a diagnosis. Before WES reanalysis was initiated, previously identified variants (variants of uncertain significance (VUS)) were first reevaluated (JWR, AH, SK, MS, EKB). If considered (likely) pathogenic, the patient was not included in this study. If the variant was still considered to be a VUS, the patient was included in the study. A second analysis was considered a reanalysis if the two analyses were performed more than two years apart, or if they were performed more than 6 months apart, but there was a specific reason for reanalysis (see Fig. 1). Patients were excluded if they were older than 18 years at initial analysis or if they did not have ID or NDD.
Demographic information, phenotypic characteristics, the presence of dysmorphic features, and genetic test specifications and outcomes were retrospectively obtained from chart review. Whether consanguinity existed was determined by anamnesis and was defined as a known common ancestor. All data were collected in an online database (CastorEDC).
To gain insight into the organization and yield of WES (re)analysis in the Netherlands, a questionnaire study was conducted among all clinical genetics departments of the academical medical centers in the Netherlands (n = 7) (for complete questionnaire translated to English, see Supplement material S1).
Variant analysis
WES-based analyses were used for all patients in the cohort (for test characteristics per patient, see Supplementary material 3). WES reanalysis was performed on existing data; no new capture was performed. At the moment of the initial diagnostic request, exome sequencing was performed on an Illumina platform after exome enrichment with the Agilent SureSelectXT Human All Exon (V5 or V7) or SureSelect Clinical Research Exome V2 kits at Genomescan B.V., Leiden, the Netherlands. Burrows-Wheeler Aligner was used for read alignment, and Genome Analysis Tool Kit was used for variant calling. For the annotation of the variants, a specific in-house developed program was used. From 2018, data analysis was performed using Moon software, Diploid, Belgium. Based on patient sex, age of onset of symptoms, Human Phenotype Ontology (HPO) terms, and sequence data, Moon software prioritizes the variants using artificial intelligence [36]. In most cases, gene panel analysis (showing variants associated with known intellectual disability genes) was performed, followed by open exome analysis (showing variants in all genes, mostly used to search for de novo variants in genes not (yet) included in the gene panels) in some cases.
The laboratory reported variants of uncertain significance (VUS) in candidate genes (genes not yet associated with NDD but having a function that may be involved in the development of NDD), VUS in known genes, likely pathogenic variants and pathogenic variants in known genes. The latter two categories were merged in this study and referred to as (likely) pathogenic variants.
Data analysis
The primary outcomes were (i) diagnostic yield at reanalysis, (ii) reasons for detecting a new possibly causal variant at reanalysis, (iii) unsolicited findings (clinically relevant findings not associated with the indication of the test), and (iv) factors associated with positive result of reanalysis. The diagnostic yield was defined as the percentage of cases for whom a (likely) pathogenic variant or VUS in a known gene with a clear link to the phenotype was identified. Factors tested for association with positive reanalysis were phenotypic and test characteristics, individual HPO terms, and disease groups based on HPO terms.
Normally distributed data were expressed by the mean and standard deviation, while skewed data were described by the median and range. To statistically compare groups, the Mann–Whitney test was conducted for continuous values and the two-sided Fisher exact test to compare the proportions within categorical variables. A p-value < 0.05 was considered statistically significant, and Bonferroni’s correction was used to correct for multiple testing [37]. All analyses were conducted using IBM SPSS Statistics version 25.
Results
Demographics
One hundred and fifty-nine patients were included in the LUMC: 63 females and 96 males (Table 1). Median age of the patients at the time of the initial analysis was 7 years (range 1 day–17 years). All had NDD, 54.4% of the patients had ID. Twenty-five patients were offspring of consanguineous parents (15.7%). A change in phenotypic characteristics between the first and second analyses was reported in 31.4% of the children.
Initial genetic data analysis and reanalysis
The initial analyses were conducted with gene panel followed by open analysis of the complete exome as the most used strategy (67%). For the reanalyses, an HPO-based analysis in combination with a complete exome analysis was most frequently used (76%; Supplementary material S2). The mean time between the analyses was 3.7 years (range 0.5–8.4 years). In most cases, the reanalysis was initiated by the clinical geneticist (65%) or treating physician (29%; mainly pediatricians, pediatric neurologists, general practitioners, intellectual disability physicians). Parents initiated the reanalysis in 6% of cases. In 13.8% of patients, a new analysis was initiated due to the development of a new phenotypic characteristic.
HPO terms
HPO terms were registered for the patients in which HPO-based analysis was performed at reanalysis (n = 154). In total, 795 terms were used, with a median of 5.2 HPO terms per patient (range 1–19 terms). Of the 312 unique terms that were used to describe the patients, “HP:0000750 Delayed speech and language development,” “HP:0000717 Autism” and “HP:0001256 Intellectual disability, mild” were used most (Supplementary material S4).
Diagnostic yield of reanalysis
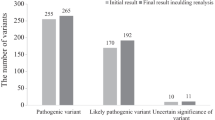
In thirty-eight patients (23.9%), a new variant was reported at reanalysis (Fig. 2). There were no unsolicited findings. A (likely) pathogenic variant in a known gene, or VUS with a clear link to the phenotype, was found in twenty cases (12.6%; Table 2). These diagnoses influenced the further treatment policy in 15 (75.0%) of these patients (family planning advice, screening plan for associated signs and symptoms, referral to a Centre of Expertise).
In 20 patients, a conclusive diagnosis was found (Table 2). A likely pathogenic variant that was clearly linked to the phenotype was found in 17 patients. In patient 7 and 24, a (likely) pathogenic variant in the FOXP1 gene was found due to an update in filtering. In patients 114 and 171, a (likely) pathogenic variant was found in the SPEN gene, of which the first gene-disease association was described in 2020, and therefore, it was not found at previous analyses. In the other patients, a variety of new (likely) pathogenic variants was found, mostly because of the discovery of new genes. A VUS was likely causal for the phenotype in three patients, based on of functional data (n = 1), and/or a clear phenotypic match. In patient 51, a homozygous missense variant was found in the OTUD6B gene. The VUS was not described previously in the medical literature, was observed only twice in control populations, was located in an evolutionarily conserved amino acid, and was predicted as pathogenic by multiple prediction programs. The phenotype of the patient matched the disease specification associated with the gene. In patient 26, a missense variant was found in the TSC2 gene. This variant was predicted as pathogenic by multiple prediction programs, was never found in control population, and was located in an evolutionarily highly conserved amino acid. TSC function and expression were reduced. This variant fitted the characteristics of the patient, who had developmental delay despite an almost normal IQ, psychiatric problems, and epilepsy. In patient 164, a frameshift variant in the TNRC6B gene was found which lead to the early introduction of a termination codon. This VUS was never found in control populations and had never been described in literature. The patient had mild ID and behavioral problems, which fit the associated characteristics of the gene.
VUS or (likely) pathogenic variants with a questionable link to the phenotype were found in four cases (2.5%). In patient 140, a likely pathogenic nonsense variant in the ZMYM2 gene was found (Supplementary material S3), for which the patient did not completely match the phenotype. In patient 5, a VUS (homozygous missense variant) in the DCHS1 gene was found, of which the patient matched the developmental delay and hearing problems associated with the gene, but not the pronounced dysmorphisms. In patient 27, a maternally inherited VUS (heterozygous splice-site variant) in the AGO1 gene was found. No inherited variants have been described before and the phenotype only matched the patient’s developmental problems. In patient 37, a (heterozygous in-frame-deletion) VUS in the SIN3A gene was found, which could be linked to the patient’s ID and behavioral problems; however, the patient had tall stature and no dysmorphisms associated with Witteveen-Kolk syndrome. Segregation analysis of the variant was not possible.
In five cases (3.1%), a VUS/(likely) pathogenic variant was found that did not explain the phenotype. In patient 75, a paternal VUS was discovered in the RAD21 gene. She did not have any phenotypical characteristics associated with the gene apart from ID and her father was healthy. A VUS in ASH1L, found in patient 30, was also present in a healthy sister. In patient 6, a normal functional metabolic test made the X-linked inherited variant in TMLHE less likely causal. In patient 115, a paternal variant in NPRL3 was found. In patient 72, the variant in the IRF2BPL gene was less likely to be pathogenic because of a mismatch with the described phenotype (severe neurological problems in early childhood, lacking at 19 years of age).
Reanalysis led to the detection of variants in candidate genes in nine patients (5.7%; Fig. 2; Supplementary material S3).
The largest proportion of new variants (12/38; 32%) was found due to recently published gene-disease associations (Fig. 3). Other major reasons for discovering new variants were updates in filtering (7/38; 18%), changes in VUS reporting (6/38; 16%), gene panel updates (4/38; 11%), and data analysis by Moon software (4/38; 11%).
Reasons for discovering the variant n = 38 at reanalysis. The number of variants that is found for a specific reason is displayed on the Y-axis. Reasons for finding new variants at reanalysis were analyzed in collaboration with a laboratory specialist and grouped into the following categories: “New gene discovery”; “Updated filtering”; Changed reporting VUS”; “Gene panel update”; Moon analysis”; More knowledge about gene”; Change patient characteristics”; “Interpretation error.” If the gene-disease association of a particular variant had been discovered at an initial analysis but had not been included in the gene panel yet, the reason for finding the variant was categorized as “Gene panel update” at the time of reanalysis. If the gene-disease association was discovered after the initial analysis, the category “New gene discovery” was used. The identification of a new variant was categorized as “Moon analysis” if Moon analysis facilitated the identification, for example, by identifying a paternal/maternal variant as a possible diagnosis. If a change in patient characteristics resulted in diagnosis, the category “Change patient characteristics” was used. Changes in the filtering could also lead to an earlier missed variant, hence the category “Updated filtering”. If a variant was missed at initial analysis because it was mistakenly interpreted by the lab specialist, it was categorized as “Interpretation error.” Some VUS were found, but not reported at initial analysis due to the reporting guidelines at that time, hence the category “Changed reporting VUS.” If the knowledge about a gene-disease association expanded leading to a new diagnosis, the category “More knowledge about gene” was used
Reinterpretation of VUS
Fifty-two patients had a VUS in a known (n = 29) or candidate (n = 23) gene at the initial analysis. By reevaluating the phenotype and the existing knowledge and reanalysis, the VUS at the initial analysis was concluded to be more likely causal in three cases (5.8%). One VUS was concluded to be causal after the discovery of ataxia at the reevaluation by the neurologist and discussions with expert-colleagues in a young patient with a de novo missense variant in SCN8A who grew into the phenotype. One patient was included in a case series after reanalysis (de novo heterozygous missense variant in GRIK2), finding a similar phenotype in other patients with missense variants in this gene. The last patient with global developmental delay, cataract, and MRI abnormalities had a de novo missense variant in the ITSN1-gene, which was concluded to be likely causal because of the presence of the specific characteristic of cataract both in the patient and in other patients with variants in the guanine nucleotide exchange factor genes.
Including these three diagnoses, the total diagnostic yield is 14.5%.
The diagnostic yield in the group of patients who had VUS at first analysis was 19.2% vs. 12.1% for the patients in whom no VUS was detected at the previous analysis (p = 0.2). In six patients, the VUS was classified as probably benign after update.
Predictors of positive reanalysis
We found a significantly higher diagnostic yield at reanalysis in patients with dysmorphic features compared to patients without dysmorphic features (p = 0.001; Table 3). No other clinical characteristics were significantly associated with diagnostic yield at reanalysis (Supplementary materials S4 and S5). There was a positive trend of microcephaly (p = 0.7) and abnormal muscle tone (p = 0.1); a negative trend was seen between autism and diagnostic yield (p = 0.3). The use of more than five HPO terms or an increased time between analyses was not associated with diagnostic yield.
In twenty-two patients, reanalysis was initiated because a new phenotypical feature was observed in the patient. In this group, the time between the two analyses was on average 1.5 years shorter than in the group that had reanalysis on another indication (p < 0.01). The diagnostic yield was 4.5% (1/22) in this group, compared to 13.9% (19/137) for patients without new clinical features (p = 0.3).
WES reanalysis policy in the Netherlands
The six other Dutch academic centers that were approached all participated in the study, and all performed WES reanalysis. In most centers, reanalysis of the data was performed after an initially negative WES, with a time interval depending on personal preference of the physician, phenotype severity, and age of the patient. Systemic reanalysis, defined as reanalysis of all previously exome negative patients, was not conducted in any center as part of routine care; two centers indicated this was mainly due to lack of capacity. Participants were generally convinced of the benefits of reanalysis in clinical practice but had concerns about the implementation. The stated concerns were related to the workload for clinical geneticists and clinical laboratory geneticists, the legal and psychological boundaries for automated reanalysis and healthcare costs.
One of the respondents, the Radboud University Medical Center, evaluated yield of reanalysis in the clinical setting. Reanalysis was performed on 329 children with neurodevelopmental disorders in whom no conclusive diagnosis was identified at initial analysis. This analysis now yielded a conclusive diagnosis in 8% (n = 26). In 16, this diagnosis was obtained due to identification of new variants, whereas in the other 10, this was based on reclassification of a previously identified VUS. In addition to new conclusive diagnoses, a possible diagnosis was obtained in 37% (n = 122 individuals, of which 49 were newly uncovered).
Discussion
This study of reanalysis of WES data in standard patient care in children with NDD had a diagnostic yield of 12.6%. The diagnostic yield in this study in daily care in a single Dutch academic hospital largely corresponds to the diagnostic yield in previously performed larger studies [9, 13, 17, 22, 32]. Consistent with previous research, the main reason for detecting a new diagnosis at WES reanalysis was the discovery of new gene-disease associations [16, 32, 38]. The genetic diagnosis had medical implications in 75% of the cases with a definitive genetic diagnosis.
VUS reclassification led to a diagnosis in three cases. This is only a minor proportion of the yield compared to a recent study, in which VUS reclassification was the main reason for finding a new diagnosis [38]. Since the current study was part of standard patient care and previously detected VUS were evaluated before the initiation of WES reanalysis and patients with (likely) causal variants were not included in this study, the reported diagnostic yield could be an underestimate of the real diagnostic yield. Also, this difference could be explained by a difference in VUS reporting guidelines of the laboratory at initial diagnosis.
Patient selection based on characteristics associated with a higher diagnostic yield has been shown to increase initial diagnostic efficiency in a large machine-learning study [35]. In our study, only the presence of dysmorphic features was significantly associated with diagnostic yield. In the study by Dingemans et al., autism was negatively associated with diagnostic yield, while microcephaly and abnormal muscle tone were positively associated with diagnostic yield [35]. Interestingly, we found similar, although non-significant, associations between autism, microcephaly, and abnormal muscle tone and diagnostic yield in our cohort. These associations should be explored further in larger cohorts to determine their value in predicting a diagnostic genetic test result.
Guidelines describing how WES reanalysis should be organized in daily practice are required to keep genetic testing available for patients with NDD. Selection of patients on the presence of specific characteristics, e.g., dysmorphic features, microcephaly, or abnormal muscle tone, or rather absence (autism), may improve the cost-effectiveness of WES reanalysis, but stringent selection will inevitably lead to underdiagnosis. Earlier studies by Schobers et al. and the Solve-RD project studied the effect of doing a systematic reanalysis in combination with ad hoc analysis and found that this increased yield by 0.6–22% [38, 39]. Therefore, we could advocate for systematic reanalysis of WES-data in all undiagnosed patients; however, there are still many judicial, practical, healthcare system, and technical difficulties to overcome before successful implementation [38]. Finally, the role of the treating pediatricians, pediatric rehabilitation specialists, and pediatric neurologists in reanalysis management has to be clearly defined to facilitate identification of undiagnosed patients with a persistent suspicion of a genetic cause of disease.
Conclusion
This study shows that WES reanalysis in standard patient care leads to a substantial increase in diagnoses in children with NDD without causing unsolicited findings. The presence of dysmorphic features was associated with a higher diagnostic yield.
Data availability
The data that support the findings of this study are available from the corresponding author, SK, upon reasonable request.
Abbreviations
- CNV:
-
Copy number variant
- HPO :
-
Human Phenotype Ontology
- ID :
-
Intellectual disability
- LUMC :
-
Leiden University Medical Centre
- NDD :
-
Neurodevelopmental disorder
- NGS :
-
Next-generation sequencing
- TIQ :
-
Total intelligence quotient
- VUS :
-
Variant of unknown significance
- WES :
-
Whole-exome sequencing
References
Maulik PK et al (2011) Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil 32(2):419–436
Leonard H, Wen X (2002) The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment Retard Dev Disabil Res Rev 8(3):117–134
Ropers HH (2010) Genetics of early onset cognitive impairment. Annu Rev Genomics Hum Genet 11:161–187
Polder JJ et al (2002) Healthcare costs of intellectual disability in the Netherlands: a cost-of-illness perspective. J Intellect Disabil Res 46(Pt 2):168–178
Vasudevan P, Suri M (2017) A clinical approach to developmental delay and intellectual disability. Clin Med (Lond) 17(6):558–561
Gil JD et al (2020) Early childhood suspected developmental delay in 63 low- and middle-income countries: Large within- and between-country inequalities documented using national health surveys. J Glob Health 10(1):010427
Miclea D et al (2015) Genetic testing in patients with global developmental delay / intellectual disabilities. A review Clujul Med 88(3):288–292
Deciphering Developmental Disorders S (2017) Prevalence and architecture of de novo mutations in developmental disorders. Nature 542(7642):433–438
Al-Nabhani M et al (2018) Reanalysis of exome sequencing data of intellectual disability samples: Yields and benefits. Clin Genet 94(6):495–501
Xiang JDY, Yang F, Gao A, Zhang W, Tang H, Mao J, He Q, Zhang Q, Wang T (2021) Genetic analysis of children with unexplained developmental delay and/or intellectual disability by whole-exome sequencing. Front Genet 12:738561
Manickam K et al (2021) Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med 23(11):2029–2037
Srivastava S et al (2019) Meta-analysis and multidisciplinary consensus statement: exome sequencing is a first-tier clinical diagnostic test for individuals with neurodevelopmental disorders. Genet Med 21(11):2413–2421
Wright CF et al (2018) Making new genetic diagnoses with old data: iterative reanalysis and reporting from genome-wide data in 1,133 families with developmental disorders. Genet Med 20(10):1216–1223
Wallace SE, Bean LJH (2017). Educational materials — genetic testing: current approaches. GeneReviews®. https://www.ncbi.nlm.nih.gov/books/NBK279899/. Accessed 30 Jun 2023
Wenger AM et al (2017) Systematic reanalysis of clinical exome data yields additional diagnoses: implications for providers. Genet Med 19(2):209–214
Fung JLF et al (2020) A three-year follow-up study evaluating clinical utility of exome sequencing and diagnostic potential of reanalysis. NPJ Genom Med 5(1):37
Nambot S et al (2018) Clinical whole-exome sequencing for the diagnosis of rare disorders with congenital anomalies and/or intellectual disability: substantial interest of prospective annual reanalysis. Genet Med 20(6):645–654
Jalkh N et al (2019) The added value of WES reanalysis in the field of genetic diagnosis: lessons learned from 200 exomes in the Lebanese population. BMC Med Genomics 12(1):11
Ewans LJ et al (2018) Whole-exome sequencing reanalysis at 12 months boosts diagnosis and is cost-effective when applied early in Mendelian disorders. Genet Med 20(12):1564–1574
Baldridge D et al (2017) The Exome Clinic and the role of medical genetics expertise in the interpretation of exome sequencing results. Genet Med 19(9):1040–1048
Eldomery MK et al (2017) Lessons learned from additional research analyses of unsolved clinical exome cases. Genome Med 9(1):26
Li J et al (2019) Reanalysis of whole exome sequencing data in patients with epilepsy and intellectual disability/mental retardation. Gene 700:168–175
Liu P et al (2019) Reanalysis of clinical exome sequencing data. N Engl J Med 380(25):2478–2480
Salfati EL et al (2019) Re-analysis of whole-exome sequencing data uncovers novel diagnostic variants and improves molecular diagnostic yields for sudden death and idiopathic diseases. Genome Med 11(1):83
Schmitz-Abe K et al (2019) Unique bioinformatic approach and comprehensive reanalysis improve diagnostic yield of clinical exomes. Eur J Hum Genet 27(9):1398–1405
Shashi V et al (2019) A comprehensive iterative approach is highly effective in diagnosing individuals who are exome negative. Genet Med 21(1):161–172
Smith ED et al (2017) Classification of genes: standardized clinical validity assessment of gene-disease associations aids diagnostic exome analysis and reclassifications. Hum Mutat 38(5):600–608
Stark Z et al (2017) Prospective comparison of the cost-effectiveness of clinical whole-exome sequencing with that of usual care overwhelmingly supports early use and reimbursement. Genet Med 19(8):867–874
Xiao B et al (2018) Marked yield of re-evaluating phenotype and exome/target sequencing data in 33 individuals with intellectual disabilities. Am J Med Genet A 176(1):107–115
Epilepsy Genetics I (2019) The Epilepsy Genetics Initiative: systematic reanalysis of diagnostic exomes increases yield. Epilepsia 60(5):797–806
Basel-Salmon L et al (2019) Improved diagnostics by exome sequencing following raw data reevaluation by clinical geneticists involved in the medical care of the individuals tested. Genet Med 21(6):1443–1451
Dai P et al (2022) Recommendations for next generation sequencing data reanalysis of unsolved cases with suspected Mendelian disorders: a systematic review and meta-analysis. Genet Med 24(8):1618–1629
Tan NB et al (2020) Evaluating systematic reanalysis of clinical genomic data in rare disease from single center experience and literature review. Mol Genet Genomic Med 8(11):e1508
Stark Z et al (2019) Does genomic sequencing early in the diagnostic trajectory make a difference? A follow-up study of clinical outcomes and cost-effectiveness. Genet Med 21(1):173–180
Dingemans AJM et al (2022) Phenotype based prediction of exome sequencing outcome using machine learning for neurodevelopmental disorders. Genet Med 24(3):645–653
O’Brien TD et al (2022) Artificial intelligence (AI)-assisted exome reanalysis greatly aids in the identification of new positive cases and reduces analysis time in a clinical diagnostic laboratory. Genet Med 24(1):192–200
Curtin F, Schulz P (1998) Multiple correlations and Bonferroni’s correction. Biol Psychiatry 44(8):775–777
Schobers G et al (2022) Reanalysis of exome negative patients with rare disease: a pragmatic workflow for diagnostic applications. Genome Med 14(1):66
Denomme-Pichon AS et al (2023) A Solve-RD ClinVar-based reanalysis of 1522 index cases from ERN-ITHACA reveals common pitfalls and misinterpretations in exome sequencing. Genet Med 25(4)
Acknowledgements
We would like to thank Q. Waisfisz (Amsterdam UMC), S. Koning (RadboudUMC), K.L.I. Gassen (UMC Utrecht), V.J.M. Verhoeven, J.J. Saris, H.T. Brüggenwirth, E.H. Hoefsloot (Erasmus MC), E.H. Gerkes, K. Abbott (UMCG) A.P.A. Stegmann, and M. Sinnema (MUMC +) for their contribution by responding to the survey.
Funding
European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability ERN-ITHACA. [EU Framework Partnership Agreement ID 3HP-HP-FPA ERN − 01–2016/739516], 3HP-HP-FPA ERN − 01–2016/739516, 3HP-HP-FPA ERN − 01–2016/739516, 3HP-HP-FPA ERN − 01–2016/739516, 3HP-HP-FPA ERN − 01–2016/739516, 3HP-HP-FPA ERN − 01–2016/739516, European Unions Horizon 2020 research and innovation program, 779257.
Author information
Authors and Affiliations
Contributions
A.v.H. E.K.B., M.S., J.W.R., E.A.R.N., C.A.L.R, and S.K. were involved in the data collection in the LUMC. L.E.L.M.V. analyzed the data of the Radboudumc. M.S. and S.K. prepared the first version of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study is not included within the scope of the WMO since individuals are not subjected to actions or have rules of conduct imposed on them due to this research (assessed by the METC Leiden-Delft-Den Haag, reference number: G20.125).
Consent to participate
No formal informed consent was required as this was a retrospective chart study. Patients/parents are aware that data may be used for research purposes, as this is stated in a summary letter sent after the genetic analysis. In the same letter, directions for opt-out are given.
Dual publication
The results/data/figures in this manuscript have not been published elsewhere, nor are they under consideration (from you or one of your contributing authors) by another publisher.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Slobbe, M., van Haeringen, A., Vissers, L.E.L.M. et al. Reanalysis of whole-exome sequencing (WES) data of children with neurodevelopmental disorders in a standard patient care context. Eur J Pediatr 183, 345–355 (2024). https://doi.org/10.1007/s00431-023-05279-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05279-4