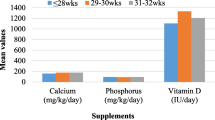
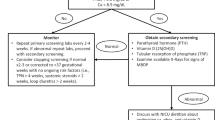
Abstract
Very low birth weight (VLBW) neonates present a high risk of metabolic bone disease (MBD). Our main objective was to determine the easiest way to make an early diagnosis of this disease by identifying surrogate biomarkers before any radiological signs occurred. We conducted in our NICU a 6-month observational prospective study, with inclusion of all singleton VLBW neonates. We collected clinical and biological data, and nutritional intakes during hospitalization. We defined biological MBD (bMBD) as alkaline phosphatase (ALP) levels superior to 600 UI/L at day of life 30 (DOL30) and performed a case–control analysis. Nine out of 30 patients (30%) exhibited bMBD. All have extremely low birth weight and were significantly younger in gestational age (GA) and smaller at birth. There was no statistically significant difference in nutritional intake between bMBD and control groups. In the bMBD group, phosphatemia was lower since DOL3. ALP was already significantly higher at DOL15, and way beyond normal range.
Conclusions: Our results showed that even the strict respect of nutritional guidelines cannot completely prevent bMBD in high-risk patients and suggest that an early screening from DOL15, with ALP levels greater than 500 UI/L, could be sufficient for detection of upcoming MBD.
What is Known: • Metabolic bone disease of prematurity (MBD) definition is not consensual, but biological changes appear earlier than radiological signs of rickets. • MBD management relies on biological evidence. Treatment is based on phosphate and/or calcium and calcitriol supplementation. | |
What is New: • Studying phosphocalcic biological assessment in very low birth weight neonates, we showed respect of nutritional guidelines could not protect from biological MBD. • Increase in alkaline phosphatase (ALP), about 500 UI/l at day of life 15, could be a biomarker of MBD with no need of X-ray evaluation and sufficient to begin a treatment to prevent osteopenia. |



Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kovacs CS (2014) Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol Rev 94:1143–1218
Kovacs CS (2015) Calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum Dev 91:623–628
Rowe JC, Wood DH, Rowe DW, Raisz LG (1979) Nutritional hypophosphatemic rickets in a premature infant fed breast milk. N Engl J Med 300:293–296
Rowe JC, Wood DH, Rowe DW, Raisz LG (1979) Hypophosphatemic rickets and breast milk. N Engl J Med 300:1397–1398
Chinoy A, Mughal MZ, Padidela R (2019) Metabolic bone disease of prematurity: causes, recognition, prevention, treatment and long-term consequences. Arch Dis Child Fetal Neonatal Ed 104:F560–F566
Rayannavar A, Calabria AC (2020) Screening for metabolic bone disease of prematurity. Semin Fetal Neonatal Med 25:101086
Chacham S, Pasi R, Chegondi M, Ahmad N, Mohanty SB (2020) Metabolic bone disease in premature neonates: an unmet challenge. J Clin Res Pediatr Endocrinol 12:332–339
Rustico SE, Calabria AC, Garber SJ (2014) Metabolic bone disease of prematurity. J Clin Transl Endocrinol 1:85–91
Ziegler EE, O’Donnell AM, Nelson SE, Fomon SJ (1976) Body composition of the reference fetus. Growth 40:329–341
Mihatsch W et al (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: calcium, phosphorus and magnesium. Clin Nutr Edinb Scotl 37:2360–2365
Faienza MF et al (2019) Metabolic bone disease of prematurity: diagnosis and management. Front Pediatr 7:143
Figueras-Aloy J et al (2014) Metabolic bone disease and bone mineral density in very preterm infants. J Pediatr 164:499–504
Mihatsch WA et al (2018) ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin Nutr Edinb Scotl 37:2303–2305
Agostoni C et al (2010) Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 50:85–91
Fetal Growth Restriction (2021) ACOG Practice Bulletin, Number 227. Obstet Gynecol 137:e16
Fenton TR (2003) A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr 3:13
Hung Y-L et al (2011) Serial measurements of serum alkaline phosphatase for early prediction of osteopaenia in preterm infants. J Paediatr Child Health 47:134–139
Viswanathan S et al (2014) Metabolic bone disease: a continued challenge in extremely low birth weight infants. JPEN J Parenter Enteral Nutr 38:982–990
Ukarapong S, Venkatarayappa SKB, Navarrete C, Berkovitz G (2017) Risk factors of metabolic bone disease of prematurity. Early Hum Dev 112:29–34
Zhang H et al (2021) Screening of serum alkaline ohosphatase and phosphate helps early detection of metabolic bone disease in extremely low birth weight infants. Front Pediatr 9:642158
Tinnion RJ, Embleton ND (2012) How to use... alkaline phosphatase in neonatology. Arch Dis Child Educ Pract Ed 97:157–163
Faerk J, Peitersen B, Petersen S, Michaelsen KF (2002) Bone mineralisation in premature infants cannot be predicted from serum alkaline phosphatase or serum phosphate. Arch Dis Child Fetal Neonatal Ed 87:F133-136
Llorente-Pelayo S et al (2020) Modified serum ALP values and timing of apparition of knee epiphyseal ossification centers in preterm infants with cholestasis and risk of concomitant metabolic bone disease of prematurity. Nutrients 12:E3854
Chinoy A, Mughal MZ, Padidela R (2021) Metabolic bone disease of prematurity—national survey of current neonatal and paediatric endocrine approaches. Acta Paediatr Oslo Nor 1992(110):1855–1862
Kelly A, Kovatch KJ, Garber SJ (2014) Metabolic bone disease screening practices among U.S. neonatologists. Clin Pediatr (Phila.) 53:1077–1083
Avila-Alvarez A et al (2020) Metabolic bone disease of prematurity: risk factors and associated short-term outcomes. Nutrients 12:E3786
Backström MC et al (2000) Bone isoenzyme of serum alkaline phosphatase and serum inorganic phosphate in metabolic bone disease of prematurity. Acta Paediatr Oslo Nor 1992(89):867–873
Fewtrell MS et al (2008) Quantitative ultrasound (QUS): a useful tool for monitoring bone health in preterm infants? Acta Paediatr Oslo Nor 1992(97):1625–1630
Catache M, Leone CR (2003) Role of plasma and urinary calcium and phosphorus measurements in early detection of phosphorus deficiency in very low birthweight infants. Acta Paediatr Oslo Nor 1992(92):76–80
Lee J, Park H-K, Kim JH, Choi YY, Lee HJ (2017) Bone mineral density according to dual energy X-ray absorptiometry is associated with serial serum alkaline phosphatase level in extremely low birth weight infants at discharge. Pediatr Neonatol 58:251–257
de Lange A, Maaskant JM, van Weissenbruch MM (2021) Is quantitative ultrasound a measure for metabolic bone disease in preterm-born infants? A prospective subcohort study. Eur J Pediatr 180:3009–3017
Tong L, Gopal-Kothandapani JS, Offiah AC (2018) Feasibility of quantitative ultrasonography for the detection of metabolic bone disease in preterm infants - systematic review. Pediatr Radiol 48:1537–1549
Mitchell SM et al (2009) High frequencies of elevated alkaline phosphatase activity and rickets exist in extremely low birth weight infants despite current nutritional support. BMC Pediatr 9:47
Jensen EA et al (2016) Determinants of severe metabolic bone disease in very low-birth-weight infants with severe bronchopulmonary dysplasia admitted to a tertiary referral center. Am J Perinatol 33:107–113
Chen W, Zhang Z, Dai S, Xu L (2021) Risk factors for metabolic bone disease among preterm infants less than 32 weeks gestation with bronchopulmonary dysplasia. BMC Pediatr 21:235
Visser F, Sprij AJ, Brus F (2012) The validity of biochemical markers in metabolic bone disease in preterm infants: a systematic review. Acta Paediatr Oslo Nor 1992(101):562–568
Matejek T et al (2019) Parathyroid hormone — reference values and association with other bone metabolism markers in very low birth weight infants - pilot study. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet 32:2860–2867
Czech-Kowalska J et al (2016) The clinical and biochemical predictors of bone mass in preterm infants. PLoS ONE 11:e0165727
Moreira A et al (2014) Parathyroid hormone as a marker for metabolic bone disease of prematurity. J Perinatol Off J Calif Perinat Assoc 34:787–791
Tan Y-L, Tsao P-N, Chou H-C, Yen T-A, Chen C-Y (2021) Hypophosphatemia as an early metabolic bone disease marker in extremely low-birth-weight infants after prolonged parenteral nutrition exposure. JPEN J Parenter Enteral Nutr 45:1268–1274
Motokura K et al (2021) Appropriate phosphorus intake by parenteral nutrition prevents metabolic bone disease of prematurity in extremely low-birth-weight infants. JPEN J Parenter Enteral Nutr 45:1319–1326
Christmann V et al (2016) Changes in biochemical parameters of the calcium-phosphorus homeostasis in relation to nutritional intake in very-low-birth-weight infants. Nutrients 8:E764
Author information
Authors and Affiliations
Contributions
EMS, MJ, and PB conceived and designed the study. MJ, LL, PYW, AD, EMS, and PB provided clinical data. MJ and EMS analyzed the data. EMS and MJ drafted the article, and PB revised it critically for important intellectual content. All authors contributed to the writing and approval of the final version to be published.
Corresponding author
Ethics declarations
Ethics approval
“French Advisory Committee for the Protection of People” and “Commission Nationale de l’Informatique et des Libertés” no. 2051804.
Consent to participate
Informed consent was obtained from legal guardians.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Motte-Signoret, E., Jlassi, M., Lecoq, L. et al. Early elevated alkaline phosphatase as a surrogate biomarker of ongoing metabolic bone disease of prematurity. Eur J Pediatr 182, 1829–1837 (2023). https://doi.org/10.1007/s00431-023-04839-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04839-y