Abstract
This cohort study aimed to evaluate the impact of an individualised nutritional care approach combining standardised fortification with adjustable fortification on postnatal growth and body composition in extremely low birth weight (ELBW) infants. We included ELBW infants admitted to a neonatal intensive care unit and still hospitalised at 35 weeks postmenstrual age (PMA). The fortification of human milk was standardised (multicomponent fortifier) between 70 mL/kg/day and full enteral feeding, and then individualised using adjustable fortification. When weight gain was below 20 g/kg/day, protein or energy was added when serum urea was below or above 3.5 mmol/L, respectively. Postnatal growth failure (PNGF) was defined as being small for gestational age at discharge and/or when the Z-score loss between birth and discharge was higher than 1. Body composition was assessed between 35 and 41 weeks of PMA. Among the 310 ELBW infants included, the gestational age of birth was 26.7 ± 1.8 weeks, and the birth weight was 800 ± 128 g. The mean Z-score difference between birth and discharge was moderately negative for the weight (−0.32), more strongly negative for length (−1.21), and almost nil for head circumference (+ 0.03). Only 27% of infants presented PNGF. At discharge, fat mass was 19.8 ± 3.6%. Multivariable analysis showed that the proportion of preterm formula received and gestational age at birth were independently associated with the percentage of fat mass.
Conclusion: The individualised nutritional care approach applied herein prevented postnatal weight loss in most infants, limited length growth deficit, and supported excellent head circumference growth.
What is Known: • At least half of extremely low birth weight infants are small for gestational age at discharge and postnatal growth deficit has been associated with impaired neurocognitive and renal development. • Human milk is the main milk used in neonatology and, although fortification of human milk is a standard of care, there is no consensus regarding the optimal fortification strategy to be adopted. | |
What is New: • Using an approach combining standardised fortification followed by individualised adjustable fortification limited postnatal growth deficit for body weight and head circumference. Postnatal growth failure is not a fatality in extremely low birth weight infants. • Each additional gestational age week at birth resulted in a decrease in fat mass percentage at discharge, which was higher than in foetuses of the same gestational age, likely representing a necessary adaptation to extra-uterine life. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postnatal growth failure, which is associated with impaired neurocognitive and renal development [1,2,3,4], was observed in nearly 100% of very low birth weight infants at the end of the 1990s and still occurs in more than half of these infants [5]. In the Swedish EXPRESS cohort of ELBW infants, the median Z-scores for body weight were −0.66 at birth and −1.84 at 36 weeks PMA. Importantly, 44% of these infants had a Z-score below -2 standard deviation (SD) at discharge [6]. Although length growth deficit is also very common, the vast majority of children gradually catch up between the ages of 2 and 8 years and are within normal height ranges as adults [7, 8]. A deficit in fat-free mass (FFM) at discharge has been associated with suboptimal neurological outcomes, and the proportion of fat mass (FM) is known to be higher in premature infants compared to foetuses of the same gestational age (GA) [9, 10].
Human milk fortification strategies used in neonatal units worldwide are highly variable [11, 12]. Individualised fortification, whether adjustable or targeted, achieves better postnatal growth than standardised fortification [13, 14]. Adjustable fortification consists in modulating protein enrichment according to serum urea levels, while targeted fortification is based on the analysis of breast milk to adjust protein or energy content [11, 13]. The latter is time- and resource-consuming and its superiority over adjustable fortification have not been demonstrated [14].
The aim of this study was to evaluate the frequency of postnatal growth deficit and assess body composition at discharge in ELBW infants using an individualised nutritional care approach combining standardised fortification followed by adjustable fortification adapted to weight gain and serum urea.
Population and methods
This single-centre retrospective observational study included infants born with a birth weight less than 1000 g, admitted within the first 24 h of life to the neonatal intensive care unit of the Croix-Rousse University Hospital in Lyon (France), and still hospitalised at 35 weeks PMA. Infants with serious congenital malformations were excluded.
Data were prospectively recorded in the patient’s electronic files (ICCA, Philips, Boblingen, Germany). Daily protein and energy intakes were assessed on the first day of each week of life and compared to recommended intakes (protein: < 1 kg: 4.0–4.5 g/kg/day, 1–1.8 kg: 3.5–4.0 g/kg/day, energy: 110–135 kcal/kg/day) [15]. Serum urea was measured weekly. Bronchopulmonary dysplasia (ventilatory support or oxygen therapy at 36 weeks PMA), intraventricular haemorrhage grade 3 or 4, periventricular leukomalacia, retinopathy of prematurity stage ≥ 3, and necrotising enterocolitis stage ≥ 2 were collected.
Body weight was measured daily during the first week of life, and then weight, crown-heel length, and head circumference (HC) were measured weekly. The length was measured using a rigid measuring board suitable for premature newborns (Premie Stadiometer, Ellard instrumentation, Monroe, USA). Anthropometric data were expressed in absolute values and Z-scores, and differences in Z-scores between birth and discharge were calculated [16]. Infants were considered to be small for GA (SGA) when the Z-score for body weight was ≤ −1.28 (10th percentile equivalent) [16]. PNGF was considered when the Z-score loss between birth and discharge was higher than 1 or when the Z-score for body weight at discharge was ≤ −1.28. Air displacement plethysmography (PEA POD®, Cosmed France, Brignais, France) was performed between 35 and 41 weeks PMA. Both FM% and absolute values of FFM were collected. Since at 35 to 41 weeks, infants were 2 to 4 months old, the data from infants born at 35–41 weeks as well as the data obtained in 2-month-old term infants were used as a reference [10].
Parenteral nutrition was started within the first 2 h of life, was individualised as soon as possible (within 48 h), and continued until the enteral ration reached 120 mL/kg/day. Enteral nutrition started on the first day of life using donor human milk. Then, the mother’s own milk was introduced as soon as possible, when available. It was pasteurised up to 32 weeks of corrected age [17]. Enteral nutrition increased daily from 15 to 20 mL/kg/day, up to 160 mL/kg/day. Energy supplement started at the end of parenteral nutrition and continued until milk intake reached 160 mL/kg/day: Liquigen® 4 g/100 mL (Nutricia, Saint Ouen, France). Fortification of human milk was started when enteral nutrition reached 70 mL/kg/day. Initially, all infants received a standardised fortification with a powder multicomponent fortifier: Fortipre® 4 g powder/100 mL (Nestlé, Noisiel, France) or Fortema® 3 g powder/100 mL + Nutriprem® 0.5 g powder/100 mL (Bledina-Danone, Limonest, France) (Table 1) [11]. Weight gain calculation and serum urea assessment were performed weekly. When weight gain was insufficient, i.e. < 20 g/kg/day, enteral intake was increased to 180 mL/kg/day. If it remained insufficient after 1 week, the fortification was individualised. Individualised adjustable fortification consisted of the addition of a protein supplement (Nutriprem® 1 g/100 mL) if serum urea was low (< 3.5 mmol/L) or an energy supplement (Liquigen®: 4 g/100 mL) if the serum urea was normal (> 3.5 mmol/L). Additional protein was reduced if serum urea was above 6.5 mmol/L. Fortification of human milk was maintained until body weight reached 1800 g. When the mother had not—or not enough—milk, donor human milk was used to complete or replace the mother’s own milk, and then, when body weight was equal to 1800 g, donor human milk was replaced by a preterm formula.
For statistical analysis, continuous variables were described by their means and SD, and comparisons were performed using Welch’s t-test or the nonparametric Mann–Whitney test. Categorical variables were described by the number of occurrences and percentages, and comparisons were performed using the chi-square test or Fisher’s exact test, as appropriate. Univariable and multivariable logistic regression analyses were performed to identify factors potentially associated with PNGF. Variables with p < 0.05 in univariable analysis were retained in the multivariable model. Results are presented as odds ratios and their 95% confidence intervals [95%CI]. The search for factors potentially associated with FM% was carried out using univariable and multivariable linear regression analyses. Variables with p < 0.05 in univariable analysis were retained in the multivariable model. Results are presented as an estimate with [95%CI]. The alpha-risk significance level for all analyses was set at 0.05. All analyses were performed using R software version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria).
The study was approved by the ethics committee Comité de Protection des Personnes Sud-Est IV (IRB: 00009118), and the institutional review board (Comité scientifique et éthique des Hospices Civils de Lyon, n°22_608) was registered in Clinicaltrials.gov (NCT02686801).
Results
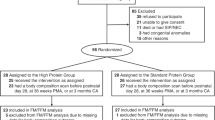
Between April 1, 2014, and December 31, 2019, 310 infants among the 490 infants with a birth weight less than 1000 g admitted to the neonatal intensive care unit were included. Body composition was assessed in 112/310 (36%) infants (Fig. 1). At birth, mean ± SD GA was 26.7 ± 1.8 weeks with a minimum of 23 weeks. The mean birth weight was 800 ± 128 g with a minimum of 440 g (Table 2).
Recommended protein intakes were reached before the end of the first week of life. Recommended energy intakes were reached at the end of the second week and were exceeded between the third and eighth week of life, bringing the protein-energy ratio slightly lower than recommended (Fig. 2). Mean serum urea levels were 4.2 ± 3.3 mmol/L at 1 month of life, 3.8 ± 2.4 mmol/L at 6 weeks of life, and 3.5 ± 1.9 mmol/L at discharge. The threshold value of 6.5 mmol/L was exceeded in 14.8% of infants at 1 month, 8.2% at 6 weeks, and 6.0% at discharge.
At birth, the mean Z-score was −0.39 for body weight, −0.44 for length, and −0.26 for HC. The mean initial weight loss was 10 ± 4% of birth weight (Table 3). Thereafter, weight gain in both boys and girls closely followed the reference curves (Fig. 3). At 1 month of life, 62 infants (20.0%) had a body weight < 10th percentile. The mean Z-score difference between birth and discharge was almost nil for HC (0.03 ± 1.12), moderately negative for the weight (−0.32 ± 0.75), and more strongly negative for length (−1.21 ± 0.92) (Fig. 4). Overall, 84 infants (27.1%) had a body weight < 10th percentile and 26 (8.4%) had a Z-score for body weight below the 3rd percentile at discharge. A total of 114 infants (36.8%) presented PNGF (Table 3). The multivariable analysis identified SGA at birth and the use of postnatal steroids as independent risk factors for PNGF. The proportion of total milk intake as the preterm formula was a protective factor (Table 4).
Body composition assessment could not be performed in 198 infants, mainly because of ventilatory support (Fig. 1). The infants who underwent a body composition assessment had similar characteristics to the others, except for their birth weight, which was significantly higher. They were also less sick and had less PNGF (Table 5). Measurement of body composition was performed at a mean of 67 ± 15 days of life, i.e., 38 ± 1 weeks PMA. FM% was 19.8 ± 3.6% of body weight, and FFM was 2314 ± 389 g (Fig. 5). FM% tended to be lower in infants that were SGA at birth than in non-SGA infants (18.8 ± 3.6% vs. 20.1 ± 3.4% p = 0.093). FM% was significantly higher in infants with optimal growth compared to those with PNGF (20.2 ± 3.2% vs. 18.1 ± 4.5%, p = 0.036). Factors influencing FM% were the proportion of milk intake as preterm formula and GA at birth. Those influencing FFM were GA at birth and sex (Table 6).
Discussion
In the present cohort of very high-risk ELBW infants, the individualised nutritional care approach applied prevented postnatal weight loss in most infants, limited length deficit, and supported excellent HC growth.
Protein intakes were close to the recommended intakes [15]. The recommended total energy intake, which may be difficult to achieve in such extremely immature infants, was reached faster than previously reported [15, 18]. Although the protein-to-energy ratio was slightly lower than recommended due to the high energy intake, the former remained higher than previously reported [19]. The high energy intake observed is likely due to the fact that the energy supplementation required after cessation of parenteral nutrition was not stopped as soon as recommended, reflecting the difficulties in fully adhering to protocols in clinical practice. However, such intakes supported early postnatal growth, as only a fifth of infants were SGA at 1 month of life, compared to the 75% previously reported, representing a significant improvement in the prevention of initial growth deficit originally described by Embleton et al. [18, 20]. Good protein utilisation was reflected by rather low serum urea. These results suggest that the slightly excessive energy intake relative to the protein intake avoided the restriction of protein utilisation which could be related to a lack of energy. Although such intakes allowed good postnatal growth in most infants, they also likely favoured high FM% at discharge. These data advocate for close monitoring of protein and energy intakes, but also that of the protein-to-energy ratio.
The postnatal growth observed in this cohort closely followed that of foetal growth, at least for body weight and HC. The present individualised nutritional care approach helped to avoid the postnatal weight deficit as demonstrated by a Z-score loss much lower than previously reported (−1), despite the fact that the infants herein were less mature [20]. This deficit was also lower than that reported more recently by Cormack et al. (−0.48) in a similar population and even lower than the −0.7 to −1 Z-score loss recently proposed as acceptable [11, 21]. In the present cohort, there were four times less infants with a Z-score for body weight below −2 at discharge than in the EXPRESS cohort [6]. Moreover, less than a third of infants herein had a Z-score for body weight below −1.28 at discharge, which is lower than previously reported in even more mature very low birth weight infants [23]. The observed absence of postnatal HC deficit in the majority of infants herein is also noteworthy, as such a deficit has been associated with suboptimal neurological outcomes [1, 24]. In a similar population, Cormack et al. reported a higher Z-score loss of 0.82 [22]. In the EPICure cohort, ELBW infants with a significant deficit in postnatal HC growth had HC below reference values as adults [25]. Although there is no strong evidence supporting that having an HC close to the mean for GA at discharge is associated with better neurodevelopment, it seems rather reassuring for the future of these high-risk infants. The length deficit observed herein was similar to the −1.5 and −1.16 previously reported [21, 26]. Surprisingly, length data are quite rarely reported in studies assessing postnatal growth in ELBW infants [20, 27], and very few authors reported an improvement in the Z-score for length during hospitalisation [28, 29]. It is well known that the final height of premature infants is approximately 1SD lower than that of term infants [30]. Furthermore, since postnatal length growth deficit can be associated with long-term consequences such as osteoporosis, large deficits in length should be avoided as much as possible [31]. The few studies that found such positive length kinetics underlined the central role of protein intake [28, 29]. This represents another reason for optimising protein intakes and protein-to-energy ratio. In summary, and in contrary to previously reported studies, the individualised nutritional care approach used in the present cohort helped limit postnatal growth deficits [32,33,34].
Of note, PNGF might be a more relevant marker of growth deficit than just SGA at discharge [35]. When using PNGF, the postnatal growth deficits in weight, length, and HC were present in more infants than when using SGA at discharge. Neonatologists should aim to reduce the risk of PNGF rather than SGA at discharge. This study confirmed that SGA at birth and postnatal steroids are independent risk factors for PNGF and found that the proportion of milk ingested as the preterm formula was a significant protective factor of PNGF. This could be due to the fact that preterm formula, which is used to supplement or replace absent or insufficient breast milk provides, a more stable nutritional supply than fortified breast milk.
Currently, there is no consensus regarding the body composition objective at the end of hospitalisation. Due to the metabolic adaptation to extrauterine life needed to increase energy storage and improve thermoregulation, the foetal body composition cannot serve as a reference [36]. Given that full-term neonates have an FM% of around 10% at birth and 25% at 2–3 months of life [11, 37] and that the FM% at discharge in the present ELBW cohort was 20%, the objective could be between that of a 36–40 week foetus and that of a full-term infant aged 2–3 months. This relatively high FM% at discharge is similar to that observed in smaller cohorts with similar postnatal weight change but higher than the 15% reported in less immature infants with a more favourable postnatal weight change [37, 38]. Contrary to what has been reported, the results herein showed that each additional GA week at birth resulted in a decrease in FM% [39]. Thus, the more immature the infant, the higher the FM% at discharge, which could reflect an increase in fat storage due to the difficulty in maintaining well-balanced protein and energy intakes throughout hospitalisation. Nevertheless, FM% has been shown to normalise within a few months after discharge [10]. Such a transient excess in FM% could thus be useful for ELBW infants, as it may represent the “price to pay” to avoid postnatal growth deficits, particularly regarding HC.
A deficit in FFM at discharge has been associated with neurological impairment at 2 years of age [9, 40]. Herein, FFM was lower than in term infants (2.8–2.9 kg), confirming the data published by Hamatschek et al. (mean of 2.5 kg), but was 300 g higher than that reported in a very low birth weight cohort [10, 39]. However, as the FFM is expressed in absolute value, it depends directly on body weight, and it is therefore difficult to compare studies, in which nutritional care and body weight at discharge varies greatly.
A limitation of this study is its single-centre design, although this did not prevent the data from a significant number of ELBW infants to be analysed. Furthermore, it avoided the impact of potential inter-centre differences in practices other than nutritional management, which could impact postnatal growth. Moreover, only a subgroup of infants could benefit from the body composition assessment herein. They had less bronchopulmonary dysplasia and therefore, less postnatal steroid treatment. However, even though the most severely ill infants did not undergo a body composition measurement, those who had it still represented a population of very high-risk ELBW infants.
In conclusion, an individualised nutritional care approach using standardised fortification followed by adjustable fortification limited body weight and HC postnatal growth deficits. FM% was higher than that of foetuses of the same GA, possibly representing a necessary adaptation to extrauterine life. Further studies are still needed to determine the growth and body composition objectives in ELBW infants according to their impact on later development.
Data availability
Data are available upon request to the corresponding author.
Abbreviations
- ELBW:
-
Extremely low birth weight
- FFM:
-
Fat-free mass
- FM:
-
Fat mass
- GA:
-
Gestational age
- HC:
-
Head circumference
- PNGF:
-
Postnatal growth failure
- PMA:
-
Postmenstrual age
- SGA:
-
Small for gestational age
References
Raghuram K, Yang J, Church PT et al (2017) Head growth trajectory and neurodevelopmental outcomes in preterm neonates. Pediatrics 140. https://doi.org/10.1542/peds.2017-0216
Vinall J, Grunau RE, Brant R et al (2013) Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med 5:168ra8. https://doi.org/10.1126/scitranslmed.3004666
Bacchetta J, Harambat J, Dubourg L et al (2009) Both extrauterine and intrauterine growth restriction impair renal function in children born very preterm. Kidney Int 76:445–452. https://doi.org/10.1038/ki.2009.201
Ordóñez-Díaz MD, Pérez-Navero JL, Flores-Rojas K et al (2020) Prematurity with extrauterine growth restriction increases the risk of higher levels of glucose, low-grade of inflammation and hypertension in prepubertal children. Front Pediatr 8:180. https://doi.org/10.3389/fped.2020.00180
Horbar JD, Ehrenkranz RA, Badger GJ et al (2015) Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics 136:e84-92. https://doi.org/10.1542/peds.2015-0129
The EXPRESS Group (2010) Incidence of and risk factors for neonatal morbidity after active perinatal care: extremely preterm infants study in Sweden (EXPRESS): morbidity in extremely preterm infants. Acta Paediatr 99:978–992. https://doi.org/10.1111/j.1651-2227.2010.01846.x
Sakurai M, Itabashi K, Sato Y et al (2008) Extrauterine growth restriction in preterm infants of gestational age ≤32 weeks. Pediatr Int 50:70–75. https://doi.org/10.1111/j.1442-200X.2007.02530.x
Saigal S, Stoskopf B, Streiner D et al (2006) Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res 60:751–758. https://doi.org/10.1203/01.pdr.0000246201.93662.8e
Ramel SE, Gray HL, Christiansen E et al (2016) Greater early gains in fat-free mass, but not fat mass, are associated with improved neurodevelopment at 1 year corrected age for prematurity in very low birth weight preterm infants. J Pediatr 173:108–115. https://doi.org/10.1016/j.jpeds.2016.03.003
Hamatschek C, Yousuf EI, Möllers LS et al (2020) Fat and fat-free mass of preterm and term infants from birth to six months: a review of current evidence. Nutrients 12. https://doi.org/10.3390/nu12020288
Picaud J-C, Vincent M, Buffin R (2021) Human milk fortification for preterm infants: a review. World Rev Nutr Diet 122:225–247. https://doi.org/10.1159/000514744
Klingenberg C, Embleton ND, Jacobs SE et al (2012) Enteral feeding practices in very preterm infants: an international survey. Arch Dis Child Fetal Neonatal Ed 97:F56-61. https://doi.org/10.1136/adc.2010.204123
Arslanoglu S, Moro GE, Ziegler EE (2006) Adjustable fortification of human milk fed to preterm infants: does it make a difference? J Perinatol Off J Calif Perinat Assoc 26:614–621. https://doi.org/10.1038/sj.jp.7211571
Fabrizio V, Trzaski JM, Brownell EA et al (2020) Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk. Cochrane Database Syst Rev 11:CD013465. https://doi.org/10.1002/14651858.CD013465.pub2
Agostoni C, Buonocore G, Carnielli VP et al (2010) Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 50:85–91. https://doi.org/10.1097/MPG.0b013e3181adaee0
Fenton TR, Kim JH (2013) A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59. https://doi.org/10.1186/1471-2431-13-59
Picaud J-C, Houeto N, Buffin R et al (2016) Additional protein fortification is necessary in extremely low-birth-weight infants fed human milk. J Pediatr Gastroenterol Nutr 63:103–105. https://doi.org/10.1097/MPG.0000000000001142
Martin CR, Brown YF, Ehrenkranz RA et al (2009) Nutritional practices and growth velocity in the first month of life in extremely low gestational age newborns. Pediatrics 124:649–657. https://doi.org/10.1542/peds.2008-3258
Moltu SJ, Blakstad EW, Strømmen K et al (2014) Enhanced feeding and diminished postnatal growth failure in very-low-birth-weight infants. J Pediatr Gastroenterol Nutr 58:344–351. https://doi.org/10.1097/MPG.0000000000000220
Embleton NE, Pang N, Cooke RJ (2001) Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics 107:270–273. https://doi.org/10.1542/peds.107.2.270
Rochow N, Raja P, Liu K et al (2016) Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr Res 79:870–879. https://doi.org/10.1038/pr.2016.15
Cormack BE, Jiang Y, Harding JE et al (2020) Relationships between neonatal nutrition and growth to 36 weeks’ corrected age in ELBW babies-secondary cohort analysis from the provide trial. Nutrients 12. https://doi.org/10.3390/nu12030760
Griffin IJ, Tancredi DJ, Bertino E et al (2016) Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch Dis Child Fetal Neonatal Ed 101:F50–55. https://doi.org/10.1136/archdischild-2014-308095
Neubauer V, Griesmaier E, Pehböck-Walser N et al (2013) Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatr Oslo Nor 1992 102:883–888. https://doi.org/10.1111/apa.12319
Ni Y, Beckmann J, Gandhi R et al (2020) Growth to early adulthood following extremely preterm birth: the EPICure study. Arch Dis Child Fetal Neonatal Ed 105:496–503. https://doi.org/10.1136/archdischild-2019-318192
Lapointe M, Barrington KJ, Savaria M, Janvier A (2016) Preventing postnatal growth restriction in infants with birthweight less than 1300 g. Acta Paediatr Oslo Nor 1992 105:e54–59. https://doi.org/10.1111/apa.13237
Senterre T, Rigo J (2012) Reduction in postnatal cumulative nutritional deficit and improvement of growth in extremely preterm infants. Acta Paediatr Oslo Nor 1992 101:e64–70. https://doi.org/10.1111/j.1651-2227.2011.02443.x
Olsen IE, Harris CL, Lawson ML, Berseth CL (2014) Higher protein intake improves length, not weight, z scores in preterm infants. J Pediatr Gastroenterol Nutr 58:409–416. https://doi.org/10.1097/MPG.0000000000000237
Loÿs C-M, Maucort-Boulch D, Guy B et al (2013) Extremely low birthweight infants: how neonatal intensive care unit teams can reduce postnatal malnutrition and prevent growth retardation. Acta Paediatr Oslo Nor 1992 102:242–248. https://doi.org/10.1111/apa.12092
Van de Pol C, Allegaert K (2020) Growth patterns and body composition in former extremely low birth weight (ELBW) neonates until adulthood: a systematic review. Eur J Pediatr 179:757–771. https://doi.org/10.1007/s00431-019-03552-z
Cooper C, Fall C, Egger P et al (1997) Growth in infancy and bone mass in later life. Ann Rheum Dis 56:17–21. https://doi.org/10.1136/ard.56.1.17
Brion LP, Rosenfeld CR, Heyne R et al (2020) Optimizing individual nutrition in preterm very low birth weight infants: double-blinded randomized controlled trial. J Perinatol Off J Calif Perinat Assoc 40:655–665. https://doi.org/10.1038/s41372-020-0609-1
Maas C, Mathes M, Bleeker C et al (2017) Effect of increased enteral protein intake on growth in human milk-fed preterm infants: a randomized clinical trial. JAMA Pediatr 171:16–22. https://doi.org/10.1001/jamapediatrics.2016.2681
McLeod G, Sherriff J, Hartmann PE et al (2016) Comparing different methods of human breast milk fortification using measured v. assumed macronutrient composition to target reference growth: a randomised controlled trial. Br J Nutr 115:431–439. https://doi.org/10.1017/S0007114515004614
Peila C, Spada E, Giuliani F et al (2020) Extrauterine growth restriction: definitions and predictability of outcomes in a cohort of very low birth weight infants or preterm neonates. Nutrients 12:E1224. https://doi.org/10.3390/nu12051224
Sauer PJJ (2007) Can extrauterine growth approximate intrauterine growth? Should it? Am J Clin Nutr 85:608S–613S. https://doi.org/10.1093/ajcn/85.2.608S
Roggero P, Giannì ML, Amato O et al (2009) Is term newborn body composition being achieved postnatally in preterm infants? Early Hum Dev 85:349–352. https://doi.org/10.1016/j.earlhumdev.2008.12.011
Ahmad I, Nemet D, Eliakim A et al (2010) Body composition and its components in preterm and term newborns: a cross-sectional, multimodal investigation. Am J Hum Biol Off J Hum Biol Counc 22:69–75. https://doi.org/10.1002/ajhb.20955
Simon L, Frondas-Chauty A, Senterre T et al (2014) Determinants of body composition in preterm infants at the time of hospital discharge. Am J Clin Nutr 100:98–104. https://doi.org/10.3945/ajcn.113.080945
Frondas-Chauty A, Simon L, Flamant C et al (2018) Deficit of fat free mass in very preterm infants at discharge is associated with neurological impairment at age 2 years. J Pediatr 196:301–304. https://doi.org/10.1016/j.jpeds.2017.12.017
Acknowledgements
The authors would like to thank Véréna Landel (Hospices Civils de Lyon) for help in manuscript preparation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Data collection and analysis were performed by Tania Perrin, Blandine Pastor-Diez, Marion Masclef-Imbert, and Pierre Pradat. All authors interpreted the results. The first draft of the manuscript was written by Tania Perrin and Jean-Charles Picaud, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee Comité de Protection des Personnes Sud-Est IV (IRB: 00009118) and Institutional Review Board (Comité scientifique et éthique des Hospices Civils de Lyon, n°22_608).
Consent to participate
The informed consent was waived by the Institutional Review Board since this was a retrospective analysis of existing clinical data.
Consent for publication
N/A.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perrin, T., Pradat, P., Larcade, J. et al. Postnatal growth and body composition in extremely low birth weight infants fed with individually adjusted fortified human milk: a cohort study. Eur J Pediatr 182, 1143–1154 (2023). https://doi.org/10.1007/s00431-022-04775-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04775-3