Abstract
Tumor-necrosis-factor-α inhibitors (anti-TNF-α) are associated with an increased risk of tuberculosis (TB) disease, primarily due to reactivation of latent TB infection (LTBI). We assessed the performance of parallel LTBI screening with tuberculin skin test (TST) and QuantiFERON-TB Gold In-Tube assays (QFT-GIT) before anti-TNF-α treatment in children with immune-mediated inflammatory disorders in a low TB-burden setting. We conducted a multicenter cohort study involving 17 pediatric tertiary centers in Spain. LTBI was defined as the presence of a positive TST and/or QFT-GIT result without clinical or radiological signs of TB disease. A total of 270 patients (median age:11.0 years) were included, mainly with rheumatological (55.9%) or inflammatory bowel disease (34.8%). Twelve patients (4.4%) were diagnosed with TB infection at screening (LTBI, n = 11; TB disease, n = 1). Concordance between TST and QFT-GIT results was moderate (TST+/QFT-GIT+, n = 4; TST−/QFT-GIT+, n = 3; TST+/QFT-GIT-, n = 5; kappa coefficient: 0.48, 95% CI: 0.36–0.60). Indeterminate QFT-GIT results occurred in 10 patients (3.7%) and were associated with young age and elevated C-reactive protein concentrations. Eleven of 12 patients with TB infection uneventfully completed standard LTBI or TB treatment. During a median follow-up period of 6.4 years, only 2 patients developed TB disease (incidence density: 130 (95% CI: 20–440) per 100,000 person-years), both probable de novo infections.
Conclusion: A substantial number of patients were diagnosed with LTBI during screening. The dual strategy identified more cases than either of the tests alone, and test agreement was only moderate. Our data show that in children in a low TB prevalence setting, a dual screening strategy with TST and IGRA before anti-TNF-α treatment is effective.
What is Known: • The optimal screening strategy for latent tuberculosis in children with immune-mediated inflammatory disorders remains uncertain. • Children receiving anti-TNF-α drugs are at increased risk of developing severe tuberculosis disease. | |
What is New: • A dual screening strategy, using TST and an IGRA assay, identified more children with latent tuberculosis than either of the tests alone. • Identification and treatment of latent tuberculosis before initiation of anti-TNF-α therapy averted incident tuberculosis cases. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor necrosis factor-α (TNF-α) is a cytokine that plays a critical role in the formation and maintenance of granulomas, which are aggregates of macrophages and lymphocytes that contain pathogens that cannot be eliminated [1]. Upon primary infection with Mycobacterium tuberculosis (MTB), granulomas restrict the spread of MTB in the pulmonary parenchyma in most individuals, and the infection enters a stage of dormancy referred to as latent TB infection (LTBI) [1]. It has been estimated that approximately one-quarter of the world population has LTBI at present [2].
TNF-α is also implicated in the pathogenesis of several immune-mediated inflammatory disorders (IMID), including juvenile idiopathic arthritis (JIA) and inflammatory bowel disease (IBD), where high concentrations of TNF-α lead to chronic inflammation and tissue damage [3, 4]. TNF-α inhibitor therapy has changed the natural clinical course of many pediatric IMID patients, especially those with severe disease [5, 6]. Etanercept, infliximab, adalimumab, and golimumab are approved for the treatment of a range of IMID in children, and their use in other inflammatory conditions is currently being explored [7].
Patients receiving anti-TNF-α drugs are at substantially increased risk of developing mycobacterial infections, including TB disease [8]. Besides TNF-α inhibition, the IMID itself, the recent or concomitant use of other immunosuppressive drugs (especially corticosteroids), and co-existing comorbidities often contribute to increasing the risk of TB in this particular patient population. In adults on anti-TNF-α treatment TB disease usually results from LTBI reactivation, often manifesting as severe extrapulmonary or disseminated disease [7]. In contrast, in children treated with anti-TNF-α drugs TB disease typically stems from recent exposure and resulting uncontrolled primary infection [9].
The risk of LTBI reactivation has been reduced with the implementation of LTBI screening before the initiation of anti-TNF-α drugs and the provision of preventive treatment to those with positive test results [10]. Currently, there are only two types of diagnostic tests that can identify the presence of LTBI—the tuberculin skin test (TST) and interferon-gamma (IFN-γ) release assays (IGRAs). IGRAs are considered to be more specific than the TST, but have a number of disadvantages, including higher cost, the need for adequate laboratory facilities, and reduced accuracy in young children [11].
The optimal LTBI screening strategy in patients with IMID remains uncertain, and existing guidelines are based on low-quality evidence [12]. As with other immunocompromised individuals, many experts recommend a dual screening strategy, including both the TST and an IGRA test, to increase overall sensitivity [7]. However, there is considerable variation in the use of TST and IGRAs in widely used recommendations [12,13,14,15]. At present, in children and adolescents with IMID, LTBI screening recommendations prior to initiation of anti-TNF-α drugs are largely based on adult experience [16, 17].
This study aimed to assess the performance of parallel LTBI screening with TST and QuantiFERON-TB Gold In-Tube (QFT-GIT) assays before initiation of anti-TNF-α therapy in a large cohort of children and adolescents affected by IMID in a low TB burden setting.
Materials and methods
Setting and participating centers
We conducted a cohort observational multicenter study from November 2013 to September 2016 in Spain. In Spain, the incidence of TB has persistently decreased over the past 20 years to 9.3 cases/100,000 in 2019 (4.2/100,000 in individuals aged < 15 years) [18]. Bacillus Calmette-Guérin (BCG) vaccination is not part of the routine childhood immunization program in Spain, except for the Basque Country, where a single dose was given at birth until 2013. The Spanish Societies of Pediatric Rheumatology, Pediatric Gastroenterology and Pediatric Infectious Diseases, and the Spanish Pediatric TB Research Network [19, 20] provided support by encouraging their members to participate in this project. Ultimately, a total of 17 pediatric tertiary referral centers participated in the study.
Eligibility criteria and case definitions
Children and adolescents (< 18 years-of-age at inclusion) affected by IMID in whom screening for LTBI was simultaneously performed the same day with TST and QFT-GIT before treatment with anti-TNF-α drugs was initiated were eligible for inclusion and recruited consecutively. Patients with a previous history of LTBI or TB disease, those previously treated with anti-TNFα drugs, and those with other underlying diseases that increase the risk of TB (malignancy, primary immunodeficiencies, and HIV infection) were excluded from participation. LTBI was defined as the presence of any positive result in the screening tests used (i.e., positive TST and/or QFT-GIT result) in the absence of clinical or radiological signs (if performed) of TB disease. The diagnosis of TB disease was based on epidemiological, clinical, radiological, and microbiological findings according to published consensus criteria [21], irrespective of TST or QFT-GIT results. In this manuscript, the term “TB infection” is used to encompass both LTBI and TB disease.
Immunodiagnostic TB tests
TSTs were performed by intradermal injection of 0.1 mL (2 tuberculin units) of purified protein derivative (Tuberkulin PPD RT23, Statens Serum Institut, Copenhagen, Denmark), and results were read by trained personnel after 48–72 h. The cut-off for a positive TST result was defined according to national guidelines as an induration ≥ 5 mm, independent of BCG vaccination status [22]. All QFT-GIT assays were performed in fully accredited routine diagnostic laboratories at each participating center and interpreted according to manufacturer’s instructions [23]. In brief, the assay result was classified as positive if the concentration of IFN-γ in the antigen-stimulated sample was ≥ 0.35 IU/mL after subtraction of the IFN-γ concentration in the negative control sample, irrespective of the IFN-γ concentration in the positive control sample. The result was classified as negative if the background-corrected IFN-γ concentration in the antigen-stimulated sample was < 0.35 IU/mL with adequate response in the mitogen-stimulated sample (positive control ≥ 0.50 IU/mL). If the background-corrected IFN-γ concentration in the antigen-stimulated sample was < 0.35 IU/mL with a positive control response < 0.50 IU/mL or the IFN-γ concentration in the negative control was ≥ 8.0 IU/mL, the result was classified as indeterminate.
Data collection
A standardized data collection tool was designed and distributed to all co-investigators. The returned data were collated into an Excel database (Microsoft, Redmond, WA) hosted on a secure server. The following variables were collected: demographics (gender, age at IMID diagnosis, age at LTBI screening, country of birth, ethnicity), type of IMID, immunosuppressive treatment in the previous 3 months (corticosteroids, disease-modifying antirheumatic drugs (DMARD) or combined corticosteroids and DMARD), BCG vaccination status based on presence of a visible scar in the deltoid region and/or a positive history, history of TB contact, TST (in mm induration) and QFT-GIT results (positive, negative, or indeterminate), chest X-ray (CXR) findings, erythrocyte sedimentation rate (ESR; normal value < 15 mm), and C-reactive protein (CRP; normal value < 15 mg/L) levels. In patients diagnosed with LTBI or TB disease and those with indeterminate QFT-GIT results, additional information was requested from the co-investigator about further diagnostic workup, final diagnosis regarding TB, TB treatment regimen, adverse events related to TB drugs, outcome of TB infection, and subsequent anti-TNF-α treatment initiation. In the remaining of patients, co-investigators were asked to report the duration of follow-up after LTBI screening and whether or not the patient was subsequently diagnosed with TB.
Statistical methods
Quantitative variables are reported as medians and interquartile ranges (IQR) and categorical variables as proportions with 95% confidence intervals (95% CI). Bivariate analysis was performed using chi-square and Fisher’s exact tests for categorical variables, and Student’s t and ANOVA tests for quantitative variables. Non-normally distributed variables were compared with Mann–Whitney U and Kruskal–Wallis tests. Binary logistic regression modeling, in which all variables with a p-value < 0.1 in bivariate analysis were included, was used for multivariate analysis to test the effect of covariates as risk factors for presence versus absence of TB infection, and for determinate (i.e., positive or negative) versus indeterminate QFT-GIT results. The results are presented as adjusted odds ratios (aOR) with 95%CIs.
Because there is no universally agreed gold standard for LTBI diagnosis, and both TST and QFT-GIT were therefore included in the study case definition for LTBI, we were unable to determine the sensitivity and the negative predictive value of those tests, but instead evaluated the concordance between the tests. Total percentage agreement and Cohen kappa coefficient (κ) were used to quantify concordance between TST and QFT-GIT results; indeterminate QFT-GIT results were excluded from this particular analysis. Strength of agreement was defined as poor (κ ≤ 0.2), fair (0.2 < κ ≤ 0.4), moderate (0.4 < κ ≤ 0.6), good (0.6 < κ ≤ 0.8), and excellent (κ > 0.8). All statistical analyses were performed using SPSS V24 (IBM; Armond, NY), with statistical significance defined as a p-value < 0.05.
Ethics approval
The study was carried out in accordance with the Declaration of Helsinki. Ethics approval was obtained from the Hospital Sant Joan de Déu Ethics Committee (reference PIC 133-13) and from the ethics committees of every participating center thereafter. Informed consent for participation was obtained from the parents/guardians of each participant and assent in patients aged > 12 years.
Results
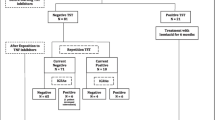
Overall, 283 patients (163 girls, 57.6%) were submitted by the participating centers, 13 of whom did not fulfill the inclusion criteria (Fig. 1). Therefore, a total of 270 patients were included in the final study population (153 girls, 56.7%). The predominant underlying IMID comprised JIA (52.2%), IBD (Crohn’s disease 23.7%; ulcerative colitis 11.1%), and idiopathic uveitis (7.4%) (Supplementary Table 1). Spain was the most common country of family origin (88.1%) and also the commonest country of birth (93.7%). Twenty-four patients (8.9%) had previously been vaccinated with BCG; 18 of those were born in Spain.
Median (IQR) age at IMID diagnosis and at LTBI screening was 8.6 (3.8–12.0) and 11.0 (6.5–13.2) years, respectively. At the initial assessment, none of the patients reported previous contact with a TB patient. In the preceding 3 months, 104 (38.5%), 22 (8.1%), and 79 (29.3%) of the participants had been treated with conventional DMARDs (other than anti-TNF-α drugs), corticosteroids, or both, respectively; only 65 (24.1%) had not received any immunosuppressant drugs during that period.
CXRs were performed in 190 (70.4%) patients, and radiological findings consistent with TB were observed in only one participant (patient 10, Table 1). Median (IQR) ESR and CRP levels were 14 (5–28) mm and 2.9 (0.7–15.9) mg/L, respectively; 51.7% (135/261) and 25.4% (68/268) of the patients had elevated ESR or CRP levels, respectively.
Overall, 12 patients (4.4%; 10 patients [3.7%] if a 10 mm TST cut-off had been used) were found to have TB infection at the initial assessment, comprising 11 patients with LTBI and one with TB disease. The latter was a 10-year-old boy who had systemic sclerosis but no TB symptoms at assessment; he had a negative TST, but a positive QFT-GIT result; CXRs showed fibro parenchymal opacities and a calcified right hilar lymph node (patient 10, Table 1). Among patients with TB infection, TST and IGRA result constellations varied widely (Table 1): TST+/QFT-GIT+, n = 4; TST−/QFT+, n = 3; and TST+/QFT-GIT−, n = 5. In bivariate analysis, only prior BCG vaccination (16.7% vs. 2.9% in unvaccinated patients; p = 0.012), non-Caucasian ethnicity (17.9% vs. 3.0% in Caucasian patients; p = 0.009), and birth abroad (33.3% vs. 2.7% in patients born in Spain; p < 0.0001) were associated with an increased risk of TB infection. In the logistic regression model, only birth abroad remained associated with an elevated risk of TB infection (aOR 20.7 (95% CI 1.3–333.2); p = 0.032).
In the whole cohort, after excluding indeterminate QFT-GIT results, concordance between TST and QFT-GIT results was only moderate (96.9%; k = 0.484, 95% CI 0.36–0.60). Indeterminate QFT-GIT results were observed in 10 patients (3.7%); all were due to insufficient responses in the positive control (mitogen) tube (Supplementary Table 2). All 10 patients had a negative TST result and an unremarkable CXR. In 4 of these patients, further TB immunodiagnostic tests were performed and yielded negative results. In bivariate analysis, a trend toward increased rates of indeterminate QFT-GIT results was observed with younger age at initial assessment (median: 7.9 years vs. 11.1 years in patients with a determinate QFT-GIT result; p = 0.095). Also, patients with indeterminate results had higher median CRP (30.8 vs. 2.9 mg/L; p = 0.027) and ESR (32 vs. 14 mm; p = 0.094) levels than those with determinate assay results. In the logistic regression model, statistical significance was maintained for younger age at initial assessment (aOR 0.838 (95% CI 0.708–0.993) per year of age increase; p = 0.042) and higher CRP levels (aOR 1.019 (95% CI 1.001–1.037) per 1 mg/L increase; p = 0.043).
Eleven out of 12 patients with TB infection received standard LTBI (n = 10) or TB (n = 1) treatment regimens [22], without major adverse events (Table 1); in the remaining patient (patient 12), a decision was made not to initiate preventive therapy, but to use close monitoring instead. Eight of the 11 patients who received anti-TB therapy were subsequently commenced on anti-TNFα treatment 0.1–1.6 years after the initial assessment. Only one patient, a 9-year-old girl with JIA (patient 3, Table 1) who was treated with a 9-month isoniazid regimen and then sequentially received etanercept and adalimumab, developed disseminated TB disease 3 years after initial assessment. The history revealed that she had recently had contact with a relative with pulmonary TB. Typing of her MTB isolates confirmed complete homology with the strain isolated from her relative, indicating that she had de novo infection rather than reactivation of LTBI [24]. Anti-TNF-α drugs were also started in 9 out of 10 patients with indeterminate QFT-GIT results (ranging from 0 to 1.6 years after assessment), none of whom developed TB disease during follow-up (Supplementary Table 2). Of the remaining cohort (all TST−/QFT−), only a 15-year-old boy born in Morocco who had Crohn’s disease was diagnosed with unconfirmed pulmonary TB while receiving adalimumab, 5 years after baseline assessment. He reported traveling twice to Morocco after the initial screening; no other risk factors for TB infection were identified. At TB diagnosis, repeat TST and a QuantiFERON-TB Gold Plus assay were negative. He showed good clinical and radiological response to 6-month standard anti-TB treatment. Overall, after a median follow-up period of 6.4 years, the incidence density of TB disease in this cohort was 130 (95% CI: 20–440) per 100,000 person-year.
Discussion
Our study shows that a dual LTBI screening strategy using both TST and an IGRA assay in children and adolescents prior to initiation of anti-TNF-α treatment is effective. Even though Spain is a low TB prevalence country, close to 1 in 20 patients had at least one positive immunodiagnostic test result at baseline screening, suggesting they had LTBI. Crucially, detection of LTBI in those individuals facilitated targeted preventive therapy to avert future progression to TB disease. During a median follow-up of more than 6 years, only two (0.7%) of the 270 study participants subsequently developed TB disease, equating to an observed incidence density of 130 per 100,000 person-year. In the first of those patients (the child with JIA), the history and laboratory findings make de novo infection years after LTBI screening, rather than reactivation of LTBI, highly likely. The second patient (with Crohn’s disease) presented more than 5 years after LTBI screening. This substantial delay indicates that this was also likely to be a de novo infection, as published data show that LTBI reactivation almost invariably occurs within the first 2 years of starting anti-TNF-α treatment [9].
Importantly, TB disease in children receiving anti-TNF-α therapy almost universally manifests as severe and often disseminated disease. In the largest pediatric case series to date, which comprises a total of 19 patients treated with anti-TNF-α drugs who developed TB disease, all patients presented with pre-defined severe disease, and one was diagnosed only at a post-mortem examination [9]. Of the 18 patients who were alive at presentation, 14 had miliary TB, and 4 had central nervous system involvement, both of which are associated with high levels of morbidity and mortality [25, 26]. This predilection for severe TB manifestations in children on anti-TNF-α treatment is further documented by several case reports and small case series [27, 28]. Notably, the aforementioned study also showed those children typically require prolonged TB treatment (median duration: 50 weeks), and that the risk of long-term sequelae in this patient population is substantial (observed in 28% of the survivors).
The growing number of indications of anti-TNF-α agents in the pediatric age group, the rising incidence of IBD in newly-industrialized countries [29], the availability of affordable and therapeutically equivalent biosimilars, and the recent inclusion of anti-TNF-α drugs into the WHO Essential Medicines for Children List for the treatment of JIA and Crohn’s disease (available at: https://list.essentialmeds.org/) make a sustained global increase in the use of those drugs in children and adolescents likely, including in regions with high TB prevalence. Ultimately, this may result in a greater proportion of pediatric TB patients presenting with severe TB disease than previously [30].
We used a simultaneous dual LTBI screening strategy in our study, as recommended by most recent guidelines for this particular patient population [7, 15, 17]. Concordant negative results were required to exclude TB infection in those very high-risk children. IGRA assays have been shown to have a high negative predictive value in previously healthy children [31, 32], but whether this is also the case in children with IMID, including those who have not commenced immunosuppressive therapy yet, remains uncertain. Our data highlight that discordance between TST and IGRA results is common in children with IMID, with only few patients showing concordantly positive test results. This indicates that the LTBI detection yield would be substantially lower if only one of those tests was used for screening. Similar observations regarding high levels of result discordance were reported by adults [33, 34] and smaller pediatric studies (Table 2). It could be argued that in some BCG-vaccinated patients, a TST+/IGRA− result constellation may simply reflect a false-positive TST result caused by the vaccine. However, there are increasing data to suggest that a large proportion of those children do in fact have LTBI (i.e., true-positive TST and false-negative IGRA results) [39]. Given that in routine practice, there is currently no modality to distinguish between those two groups, and considering that preventive therapy is generally well-tolerated by children while the risk of severe, potentially fatal TB disease if progression occurs is substantial, we and other experts recommend to provide preventive treatment to children with a TST+/IGRA− result constellation [40].
The rate of indeterminate QFT-GIT results in our study was 3.7%, similar to the 4% rate reported by a recent meta-analysis including more than 100,000 children [41]. All indeterminate results in our cohort were due to insufficient positive control responses. In multivariate analysis, young age and increased CRP levels at assessment were associated with a higher risk of indeterminate results. This aligns with previous data showing that IGRAs perform less well at the extremes of age [42]. Furthermore, we have recently reported that both elevated CRP levels and lymphopenia are associated with indeterminate QFT Gold Plus results in a large cohort of predominantly healthy children and adolescents [43]. Underlying IMID activity has also been associated with indeterminate results, both when assessed by activity scores or by laboratory parameters [36]. Three-quarters of the patients in our cohort were already receiving conventional DMARDs and/or corticosteroids at the initial assessment; however, we did not observe a statistically significant association between previous treatment and IGRA performance in our study. Nevertheless, exposure to corticosteroids, anti-TNF-α drugs, and other immunomodulators, including cyclosporin, has previously been shown to have the potential to impair the performance of IGRAs substantially and to increase the proportion of indeterminate and also false-negative assay results [44, 45]. Therefore, initial LTBI screening should be performed at the point when an IMID diagnosis is made, before initiation of immunosuppressive treatment. In agreement with most guidelines [12, 15, 17], we also believe that initial LTBI screening should include a chest X-ray to identify radiographic evidence of prior TB, as it happened in one patient in our study.
Our observations also highlight the need to integrate specific questions about potential recent TB exposure and new risk factors for TB (e.g., travel to high TB prevalence region) into regular clinical reviews while patients are receiving anti-TNF-α agents, considering that one of the study participants who developed TB disease had known TB exposure. Furthermore, parents should be made aware of the elevated risk of TB disease and instructed to seek medical attention if a persistent cough or constitutional symptoms occur. Physicians should have a low threshold to initiate a detailed TB workup in patients with symptoms that could be consistent with TB. Currently, the role of regular LTBI screening in an asymptomatic child on anti-TNF-α therapy without new risk factors remains uncertain [46].
Our study has a number of limitations, including the absence of a universally agreed gold standard for LTBI, shared with all other immunodiagnostic TB studies. Furthermore, LTBI screening tests were not routinely repeated during follow-up, and we could not assess any information about potential result conversions and reversions. Also, we were not able to collect quantitative QFT-GIT results, which may have provided additional insights. Finally, our results cannot necessarily be extrapolated to the latest generation QFT assay, QuantiFERON-TB Gold Plus; however, an increasing number of studies suggest that its performance does not differ substantially from previous-generation QFT assays [43].
In conclusion, by use of a dual LTBI screening strategy with TST and IGRA performed in parallel, close to 1 in 20 children who were planned to be commenced on anti-TNF-α agents were found to have evidence of LTBI, despite the low TB prevalence setting. Result concordance between both tests was only moderate, highlighting that the detection yield would be substantially lower if only a single immunodiagnostic test was to be used. Identification of children with LTBI allowed targeted preventive therapy before initiation of anti-TNF-α therapy to avert future progression to TB disease. Only two children developed TB disease during follow-up. In both de novo infection was far more likely to be the cause than LTBI reactivation, indicating that the screening and treatment strategies employed in this study were effective.
Availability of data and material
The data underlying this article will be shared on reasonable request to the corresponding author.
Code availability
Not applicable.
Abbreviations
- anti-TNF-α:
-
Tumor-necrosis-factor-α inhibitors
- aOR:
-
Adjusted odds ratio
- BCG:
-
Bacillus Calmette-Guérin
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- CXR:
-
Chest X-ray
- DMARD:
-
Disease-modifying antirheumatic drugs
- ESR:
-
Erythrocyte sedimentation rate
- IBD:
-
Inflammatory bowel disease
- IGRAs:
-
Interferon-gamma release assays
- IMID:
-
Immune-mediated inflammatory disorders
- IFN-γ:
-
Interferon-gamma
- IQR:
-
Interquartile range
- JIA:
-
Juvenile idiopathic arthritis
- κ:
-
Cohen kappa coefficient
- LTBI:
-
Latent tuberculosis infection
- MTB:
-
Mycobacterium tuberculosis
- QFT-GIT:
-
QuantiFERON-TB Gold In-Tube
- TB:
-
Tuberculosis
- TST:
-
Tuberculin skin test
References
Robert M, Miossec P (2021) Reactivation of latent tuberculosis with TNF inhibitors: Critical role of the beta 2 chain of the IL-12 receptor. Cell Mol Immunol 18:1644–1651. https://doi.org/10.1038/s41423-021-00694-9
Getahun H, Chaisson RE, Raviglione M (2015) Latent Mycobacterium tuberculosis infection. N Engl J Med 373:1179–1180. https://doi.org/10.1056/NEJMc1508223
de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ (2007) Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis 66:589–598. https://doi.org/10.1136/ard.2006.061853
Petagna L, Antonelli A, Ganini C, Bellato V, Campanelli M, Divizia A et al (2020) Pathophysiology of Crohn’s disease inflammation and recurrence. Biol Direct 15:23. https://doi.org/10.1186/s13062-020-00280-5
Blazina Š, Markelj G, Avramovič MZ, Toplak N, Avčin T (2016) Management of juvenile idiopathic arthritis: a clinical guide. Paediatr Drugs 18:397–412. https://doi.org/10.1007/s40272-016-0186-0
Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC et al (2014) Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 8:1179–1207. https://doi.org/10.1016/j.crohns.2014.04.005
Baddley JW, Cantini F, Goletti D, Gómez-Reino JJ, Mylonakis E, San-Juan R, Fernández-Ruiz M, Torre-Cisneros J (2018) ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules: Anti-tumor necrosis factor-α agents). Clin Microbiol Infect 24:S10–S20. https://doi.org/10.1016/j.cmi.2017.12.025
Zhang Z, Fan W, Yang G, Xu Z, Wang J, Cheng Q, Yu M (2017) Risk of tuberculosis in patients treated with TNF-α antagonists: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 7:e012567. https://doi.org/10.1136/bmjopen-2016-012567
Noguera-Julian A, Calzada-Hernández J, Brinkmann F, Basu Roy R, Bilogortseva O, Buettcher M et al (2020) Tuberculosis disease in children and adolescents on therapy with anti-tumor necrosis factor-alpha agents: a collaborative, multi-centre ptbnet study. Clin Infect Dis 71:2561–2569. https://doi.org/10.1093/cid/ciz1138
Toussi SS, Pan N, Walters HM, Walsh TJ (2013) Infections in children and adolescents with juvenile idiopathic arthritis and inflammatory bowel disease treated with tumor necrosis factor-α inhibitors: Systematic review of the literature. Clin Infect Dis 57:1318–1330. https://doi.org/10.1093/cid/cit489
Velasco-Arnaiz E, Soriano-Arandes A, Latorre I, Altet N, Domínguez J, Fortuny C, Monsonís M, Tebruegge M, Noguera-Julian A (2018) Performance of tuberculin skin tests and interferon-γ release assays in children younger than 5 years. Pediatr Infect Dis J 37:1235–1241. https://doi.org/10.1097/INF.0000000000002015
Hasan T, Au E, Chen S, Tong A, Wong G (2018) Screening and prevention for latent tuberculosis in immunosuppressed patients at risk for tuberculosis: a systematic review of clinical practice guidelines. BMJ Open 8:e022445. https://doi.org/10.1136/bmjopen-2018-022445
National Institute for Health and Care Excellence (2016) Tuberculosis [NG33]. Available from: https://www.nice.org.uk/guidance/ng33/chapter/recommendations#preventing-infection-in-specific-settings. Accessed 15 Mar 2022
Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E et al (2017) Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis 64:111–115. https://doi.org/10.1093/cid/ciw694
Viladrich IM, Tello ED, Solano-López G, Longo FJL, Samso CT, Martínez PS et al (2016) Consensus document on prevention and treatment of tuberculosis in patients for biological treatment. Arch Bronconeumol 52:36–45. https://doi.org/10.1016/j.arbres.2015.04.016
American Academy of Pediatrics. Committee on Infectious Diseases. Tuberculosis. In: Red book 2021–2024: report of the Committee on Infectious Diseases. 32nd Edition (2021), 786–814. Elk Grove Village, IL.
Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ et al (2010) The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J 36:1185–1206. https://doi.org/10.1183/09031936.00028510
Grupo de trabajo Plan Prevención y Control de la Tuberculosis (2019) Plan para la prevención y control de la tuberculosis en España. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad, Consumo y Bienestar Social. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/PlanTuberculosis/IND_SEG_PLAN_TB_2019_provisionales_web.pdf. Accessed 15 Mar 2022
Martínez-Planas A, Baquero-Artigao F, Santiago B, Fortuny C, Méndez-Echevarría A, Del Rosal T et al (2021) Interferon-gamma release assays differentiate between Mycobacterium avium complex and tuberculous lymphadenitis in children. J Pediatr 236:211–218. https://doi.org/10.1016/j.jpeds.2021.05.008
Soler-Garcia A, Gamell A, Santiago B, Monsonís M, Calvo C, Cobo E et al (2020) Diagnostic accuracy of QuantiFERON-TB Gold Plus assays in children and adolescents with tuberculosis disease. J Pediatr 223:212–215. https://doi.org/10.1016/j.jpeds.2020.02.025
Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M et al (2012) Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis 205:S199–S208. https://doi.org/10.1093/infdis/jis008
Moreno-Pérez D, Andrés Martín A, Altet Gómez N, Baquero-Artigao F, Escribano Montaner A, Durán DGP et al (2010) Diagnosis of tuberculosis in pediatrics. Consensus document of the Spanish Society of Pediatric Infectology (SEIP) and the Spanish Society of Pediatric Pneumology (SENP). An Pediatr (Barc) 73:143.e1–143.e14. https://doi.org/10.1016/j.anpedi.2009.12.017
QuantiFERON®-TB Gold Plus (QFT®-Plus) ELISA Package Insert (2019) Cellestis/Qiagen, Australia. Available from: https://www.quantiferon.com/wp-content/uploads/2020/01/L1083163-R06-QF-TB-Gold-Plus-ELISA-IFU-CE.pdf. Accessed 15 Mar 2022
Guerrero-Laleona C, Calzada-Hernández J, Bustillo-Alonso M, Gil-Albarova J, Medrano-San Ildefonso M, Iglesias-Jiménez E, Noguera-Julian A (2017) Disseminated tuberculosis resulting from reinfection in a pediatric patient sequentially treated with etanercept and adalimumab. Pediatr Infect Dis J 36:109–110. https://doi.org/10.1097/INF.0000000000001360
Roy RB, Thee S, Blázquez-Gamero D, Falcón-Neyra L, Neth O, Noguera-Julian A et al (2020) Performance of immune-based and microbiological tests in children with tuberculosis meningitis in Europe: a multicentre Paediatric Tuberculosis Network European Trials Group (ptbnet) study. Eur Respir J 56:1902004. https://doi.org/10.1183/13993003.02004-2019
Thee S, Roy RB, Blázquez-Gamero D, Falcón-Neyra L, Neth O, Noguera-Julian A et al (2021) Treatment and outcome in children with tuberculous meningitis – a multi-centre Paediatric Tuberculosis Network European Trials Group study. Clin Infect Dis 75:372–381. https://doi.org/10.1093/cid/ciab982
Bal ZS, Yazici P, Sen S, Eraslan C, Cavusoglu C, Karapinar B, Vardar F (2017) A fatal case of tuberculous meningitis in a child with juvenile idiopathic arthritis: a diagnostic challenge. Rev Soc Bras Med Trop 50:709–711. https://doi.org/10.1590/0037-8682-0410-2016
Ozere I, Santere R, Skangale A (2018) Development of tuberculosis in child during treatment with tumour necrosis factor-alpha inhibitor agent: could this have been prevented? Biomed J Sci Tech Res 7:5696–5698. https://doi.org/10.26717/BJSTR.2018.07.001452
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI et al (2017) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390:2769–2778. https://doi.org/10.1016/S0140-6736(17)32448-0
Nolt D (2020) Granulomas and guidelines: the search for tuberculosis during tumor necrosis factor-α inhibition. Clin Infect Dis 71:2570–2571. https://doi.org/10.1093/cid/ciz1139
Ahmed A, Feng PI, Gaensbauer JT, Reves RR, Khurana R, Salcedo K et al (2020) Interferon-γ release assays in children <15 years of age. Pediatrics 145:e20191930. https://doi.org/10.1542/peds.2019-1930
Grinsdale JA, Islam S, Tran OC, Ho CS, Kawamura LM, Higashi JM (2016) Interferon-gamma release assays and pediatric public health tuberculosis screening: the San Francisco Program Experience 2005 to 2008. J Pediatric Infect Dis Soc 5:122–130. https://doi.org/10.1093/jpids/piu119
Hsia EC, Schluger N, Cush JJ, Chaisson RE, Matteson EL, Xu S, Beutler A, Doyle MK, Hsu B, Rahman MU (2012) Interferon-γ release assay versus tuberculin skin test prior to treatment with golimumab, a human anti-tumor necrosis factor antibody, in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis. Arthritis Rheum 64:2068–2077. https://doi.org/10.1002/art.34382
Kleinert S, Tony HP, Krueger K, Detert J, Mielke F, Rockwitz K et al (2012) Screening for latent tuberculosis infection: Performance of tuberculin skin test and interferon-γ release assays under real-life conditions. Ann Rheum Dis 71:1791–1795. https://doi.org/10.1136/annrheumdis-2011-200941
Camlar SA, Makay B, Appak O, Appak YC, Esen N, Günay T, Anal O, Unsal E (2011) Performance of tuberculin skin test and interferon gamma assay for the diagnosis of latent tuberculosis infection in juvenile idiopathic arthritis. Clin Rheumatol 30:1189–1193. https://doi.org/10.1007/s10067-011-1724-3
Hradsky O, Ohem J, Zarubova K, Mitrova K, Durilova M, Kotalova R, Nevoral J, Zemanova I, Dryak P, Bronsky J (2014) Disease activity is an important factor for indeterminate interferon-γ release assay results in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 58:320–324. https://doi.org/10.1097/MPG.0000000000000205
Marino A, Chiappini E, Cimaz R, Simonini G (2017) Prebiologic therapy tuberculosis screening experience in a pediatric rheumatology center: TST and IGRA are both necessary. Pediatr Infect Dis J 36:440–441. https://doi.org/10.1097/INF.0000000000001466
Gİrİt S, Ayzit Atabek A, Şenol E, Kizilirmak TK, Pekcan S, Göktaş Ş, Öktem S, Kasapçopur Ö, Çokuğraş H (2019) Screening for latent tuberculosis in children with immune-mediated inflammatory diseases treated with anti-tumor necrosis factor therapy: Comparison of tuberculin skin and T-SPOT tuberculosis tests. Arch Rheumatol 35:20–28. https://doi.org/10.5606/ArchRheumatol.2020.7294
Tebruegge M, Dutta B, Donath S, Ritz N, Forbes B, Camacho-Badilla K et al (2015) Mycobacteria-specific cytokine responses detect tuberculosis infection and distinguish latent from active tuberculosis. Am J Respir Crit Care Med 192:485–499. https://doi.org/10.1164/rccm.201501-0059OC
Connell TG, Tebruegge M, Ritz N, Bryant P, Curtis N (2011) The potential danger of a solely interferon-gamma release assay-based approach to testing for latent Mycobacterium tuberculosis infection in children. Thorax 66:263–264. https://doi.org/10.1136/thx.2010.143396
Meier NR, Volken T, Geiger M, Heininger U, Tebruegge M, Ritz N (2019) Risk factors for indeterminate interferon-gamma release assay for the diagnosis of tuberculosis in children-a systematic review and meta-analysis. Front Pediatr 7:208. https://doi.org/10.3389/fped.2019.00208
Tebruegge M, de Graaf H, Sukhtankar P, Elkington P, Marshall B, Schuster H, Patel S, Faust SN (2014) Extremes of age are associated with indeterminate QuantiFERON-TB gold assay results. J Clin Microbiol 52:2694–2697. https://doi.org/10.1128/JCM.00814-14
Soler-Garcia A, Gamell A, Pérez-Porcuna T, Soriano-Arandes A, Santiago B, Tortola T et al (2021) Performance of QuantiFERON-TB Gold Plus assays in children and adolescents at risk of tuberculosis: a multi-center cross-sectional study. Thorax, online ahead of print. https://doi.org/10.1136/thoraxjnl-2021-217592
Clifford V, Zufferey C, Germano S, Ryan N, Leslie D, Street A, Denholm J, Tebruegge M, Curtis N (2015) The impact of anti-tuberculous antibiotics and corticosteroids on cytokine production in QuantiFERON-TB Gold In Tube assays. Tuberculosis (Edinb) 95:343–349. https://doi.org/10.1016/j.tube.2015.02.039
Edwards A, Gao Y, Allan RN, Ball D, de Graaf H, Coelho T et al (2017) Corticosteroids and infliximab impair the performance of interferon-γ release assays used for diagnosis of latent tuberculosis. Thorax 72:946–949. https://doi.org/10.1136/thoraxjnl-2016-209397
Ardura MI, Toussi SS, Siegel JD, Lu Y, Bousvaros A, Crandall W (2016) NASPGHAN clinical report: surveillance, diagnosis, and prevention of infectious diseases in pediatric patients with inflammatory bowel disease receiving tumor necrosis factor-α inhibitors. J Pediatr Gastroenterol Nutr 63:130–155. https://doi.org/10.1097/MPG.0000000000001188
Acknowledgements
The study was supported by the Spanish Societies of Pediatric Rheumatology (SERPE), Pediatric Gastroenterology (SEGHNP) and Pediatric Infectious Diseases (SEIP), and the Spanish Pediatric TB Research Network (pTBred). The preliminary results of this work were presented at the 21st European Pediatric Rheumatology Congress (Belgrade, Serbia; September 2014; Pediatr Rheumatol Online J. 2014; 12(Suppl 1): P282), at the Annual European Congress of Rheumatology (Rome, Italy; June 2015; Ann Rheum Dis. 2015; 74(Suppl 2): THU0513), and at the 34th Annual Meeting of the European Society for Paediatric Infectious Diseases (Brighton, UK; May 2016).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by a grant Award “Moving4 niños con artritis” from the Sociedad Española de Reumatología Pediátrica (SERPE) to JCH. ANJ was supported by “Subvencions per a la Intensificació de Facultatius Especialistes” (Departament de Salut de la Generalitat de Catalunya, Programa PERIS 2016–2020) [SLT008/18/00193].
Author information
Authors and Affiliations
Contributions
JCH, JA, JMdC, CF, MT, and ANJ contributed to the study conception and design. All authors participated in patients’ enrollment and clinical follow-up. Material preparation, data collection, and analysis were performed by JCH, MT, and ANJ. The first draft of the manuscript was written by JCH and ANJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was obtained from Hospital Sant Joan de Déu (Barcelona, Spain) Ethics Committee (reference PIC 133-13).
Consent to participate and for publication
Informed consent and assent were obtained from the parents and from patients aged > 12 years, respectively.
Conflict of interest
MT has received QuantiFERON assays at reduced pricing or free of charge for other TB diagnostics projects from the manufacturer (Cellestis/Qiagen) and has received support for conference attendance from Cepheid. The rest of the authors have no other conflicts of interest to disclose. Collaborators See Supplementary Appendix 1 for the complete list of investigators.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Calzada-Hernández, J., Anton, J., Martín de Carpi, J. et al. Dual latent tuberculosis screening with tuberculin skin tests and QuantiFERON-TB assays before TNF-α inhibitor initiation in children in Spain. Eur J Pediatr 182, 307–317 (2023). https://doi.org/10.1007/s00431-022-04640-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04640-3