Abstract
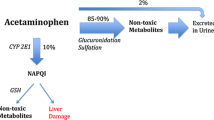
Drug-associated harm is common but difficult to detect in the hospital setting. In critically ill children, we sought to evaluate drug-associated hepatic injury following enteral acetaminophen error, defined as acetaminophen dosing that exceeds daily maximum recommendations. This retrospective cohort study took place in two pediatric intensive care units within a pediatric hospital center. The included patients are children (< 18 years of age) admitted to the pediatric and cardiac intensive care unit between January 2008 and January 2018, and receiving enteral acetaminophen. We defined acetaminophen dosing error as exceeding daily acetaminophen dosing by > 10% the upper limit of maximum recommended dose for weight and age (> 82.5 mg/kg/day or > 4400 mg/day). We included 14,146 admissions, who received 147,485 doses of acetaminophen. Acetaminophen dosing errors occurred 1 in every 9.5 patient-days on acetaminophen. ALT and AST decreased significantly over the course of ICU admission (p < 0.0001). In patients with acetaminophen errors, ALT and AST measured in the 24 to 96 h post error were not significantly different than when measured outside this window. A sensitivity analysis using > 100 mg/kg/day as the upper daily acetaminophen error cut-off did not reveal any subsequent significant increase in ALT or ALT in the 24 to 96-h post-error window, compared to measurements taken outside the window.
Conclusion: Although the administration of acetaminophen in critically ill children frequently exceeds the daily recommended limit and vigilance is needed, we did not find any associated increase in liver transaminases following acetaminophen errors.
What is Known: • Acetaminophen dosing errors are common in pediatric outpatients. • Excessive acetaminophen dosing can be associated with harm, including hepatic injury. | |
What is New: • Exceeding daily acetaminophen dosing limit occurs 1 in every 9.5 patient-days in children admitted to the critical care unit. • In patients with daily dose excess of acetaminophen, we did not find a significant increase in the measured liver enzymes in the 24 to 96 h following the overdosing. |


Similar content being viewed by others
Availability of data
Data may be available upon request.
Code availability
Coding availability in SAS may be available upon request.
Abbreviations
- ADE:
-
Adverse drug events
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CICU:
-
Cardiac intensive care unit
- GGT:
-
Gamma-glutamyl transferase
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- PICU:
-
Pediatric intensive care unit
- PRISM:
-
Pediatric risk of mortality
- SD:
-
Standard deviation
References
Mort JR, Shiyanbola OO, Ndehi LN, Xu Y, Stacy JN (2011) Opioid-paracetamol prescription patterns and liver dysfunction: a retrospective cohort study in a population served by a US health benefits organization. Drug Saf 34(11):1079–1088
Brune K, Renner B, Tiegs G (2015) Acetaminophen/paracetamol: a history of errors, failures and false decisions. Eur J Pain 19(7):953–965
Locci C, Cuzzolin L, Capobianco G, Antonucci R (2021) Paracetamol overdose in the newborn and infant: a life-threatening event. Eur J Clin Pharmacol 77(6):809–815
Bonafide CP, Miller JM, Localio AR, Khan A, Dziorny AC, Mai M, Stemler S, Chen W, Holmes JH, Nadkarni VM et al (2020) Association between mobile telephone interruptions and medication administration errors in a pediatric intensive care unit. JAMA Pediatr 174(2):162–169
Gaetani M, Frndova H, Seto W, Parshuram C (2017) Concurrent intravenous drug administration to critically ill children: Evaluation of frequency and compatibility. J Crit Care 41:198–203
Sharek PJ, Classen D (2006) The incidence of adverse events and medical error in pediatrics. Pediatr Clin North Am 53(6):1067–1077
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 370(9596):1453–1457
Roumeliotis N, Pullenayegum E, Rochon P, Taddio A, Parshuram C (2020) A modified Delphi to define drug dosing errors in pediatric critical care. BMC Pediatr 20(1):488
Lau E (2017) The Hospital for Sick Children’s Drug Formulary and Handbook: Wolters Kluwer Clinical Drug Information 2017
Dlugosz CK, Chater RW, Engle JP (2006) Appropriate use of nonprescription analgesics in pediatric patients. J Pediatr Health Care 20(5):316–325; quiz 326–318
Temple AR, Temple BR, Kuffner EK (2013) Dosing and antipyretic efficacy of oral acetaminophen in children. Clin Ther 35(9):1361–1375.e1361–1345
Acetaminophen (2012) LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]
Roumeliotis N, Frndova H, Pullenayegum E, Taddio A, Rochon P, Parshuram CS (2022) Dosing of enteral acetaminophen in critically ill children: a cohort study. Arch Dis Child 107(4):388–393
Camire E, Moyen E, Stelfox HT (2009) Medication errors in critical care: risk factors, prevention and disclosure. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 180(9):936–943
Dart RC, Erdman AR, Olson KR, Christianson G, Manoguerra AS, Chyka PA, Caravati EM, Wax PM, Keyes DC, Woolf AD et al (2006) Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 44(1):1–18
Dart RC, Green JL, Kuffner EK, Heard K, Sproule B, Brands B (2010) The effects of paracetamol (acetaminophen) on hepatic tests in patients who chronically abuse alcohol – a randomized study. Aliment Pharmacol Ther 32(3):478–486
Temple AR, Zimmerman B, Gelotte C, Kuffner EK (2017) Comparison of the efficacy and safety of 2 acetaminophen dosing regimens in febrile infants and children: a report on 3 legacy studies. J Pediatr Pharmacol Ther 22(1):22–32
Acknowledgements
Special thanks to local pharmacists Winnie Seto and Angela Trope for their help with dosing practice in the unit over time. Thanks to Melanie Gaetani for coding support with medication identification.
Funding
This study was not funded, but N. Roumeliotis received doctoral salary support from the Fonds de Recherche Santé- Québec, and the Canadian Critical Care Trials Group (CCCTG).
Author information
Authors and Affiliations
Contributions
Dr Roumeliotis conceptualized and designed the study, conducted the statistics, summarized results, drafted the initial manuscript, and reviewed and revised the manuscript. Dr Pullenayegum contributed to study design, analysis plan, interpretation of results, critical appraisal of intellectual content, and approved final version. Drs Taddio and Rochon contributed to study design, critically appraised it for intellectual content, and reviewed and revised the final version of manuscript. Dr Parshuram conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was obtained from The Hospital for Sick Children (#1000059340) and the University of Toronto (# 00037936) approved this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Piet Leroy
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roumeliotis, N., Pullenayegum, E., Taddio, A. et al. Liver enzymes after short-term acetaminophen error in critically ill children: a cohort study. Eur J Pediatr 181, 2943–2951 (2022). https://doi.org/10.1007/s00431-022-04502-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04502-y