Abstract
Premature infants are at high risk of haemorrhage and thrombosis. Our understanding of the differences between the neonatal and adult haemostatic system is evolving. There are several limitations to the standard coagulation tests used in clinical practice, and there is currently a lack of evidence to support many of the transfusion practices in neonatal medicine. The evaluation of haemostasis is particularly challenging in neonates due to their limited blood volume. The calibrated automated thrombogram (CAT) is a global coagulation assay, first described in 2002, which evaluates both pro- and anti-coagulant pathways in platelet-rich or platelet-poor plasma. In this review, the current applications and limitations of CAT in the neonatal population are discussed.
Conclusion: CAT has successfully elucidated several differences between haemostatic mechanisms in premature and term neonates compared with adults. Moreover, it has been used to evaluate the effect of a number of haemostatic drugs in a pre-clinical model. However, the lack of evidence of CAT as an accurate predictor of neonatal bleeding, blood volume required and the absence of an evidence-based treatment algorithm for abnormal CAT results limit its current application as a bedside clinical tool for the evaluation of sick neonates.
What is Known: • The Calibrated automated thrombogram (CAT) is a global coagulation assay which evaluates pro- and anti-coagulant pathways. • CAT provides greater information than standard clotting tests and has been used in adults to evaluate bleeding risk. | |
What is New: • This review summarises the physiological differences in haemostasis between neonates and adults described using CAT. • The haemostatic effect of several drugs has been evaluated in neonatal plasma using CAT. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Preterm infants experience haemorrhage, particularly intraventricular haemorrhage (IVH), while full-term neonates typically do not [1]. Neonates have reduced levels of coagulation factors [2, 3] and hypo-reactive platelets in vitro [4,5,6], but higher levels of von Willebrand factor (VWF) with larger polymers and a higher haematocrit help maintain haemostasis [2, 3, 7].
Prothrombin time (PT) and activated partial thromboplastin time (APTT) are the standard tests used to evaluate haemostasis. Both are prolonged in preterm infants [8, 9], although there is no correlation between PT/APTT and risk of developing IVH [8, 10, 11]. PT and APTT only evaluate time to initial clot formation, not overall clot formation [12, 13]. Moreover, standard clotting tests do not evaluate for hypercoagulability [14]. Despite these limitations, they are often used to guide transfusion of blood products to correct perceived coagulation disturbances in non-bleeding neonates.
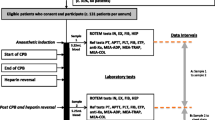
The calibrated automated thrombogram (CAT) is a global coagulation assay, which measures thrombin generation by incubating plasma with a thrombin-specific fluorogenic substrate following activation of coagulation (Fig. 1) [13]. CAT assesses overall haemostatic balance, evaluating activity of endogenous procoagulant and anticoagulant pathways, particularly important in neonates, where both are reduced [3]. CAT is performed in platelet-poor (PPP) or platelet-rich plasma (PRP). In PRP, CAT can evaluate the impact of platelet number and platelet function on thrombin generation [15].
The CAT parameters are described in Fig. 2. The lag time is the time from the beginning of the experiment until 10 nM of thrombin is produced [15]. Time to peak thrombin represents the propagation phase. Peak thrombin is the maximum amount of thrombin produced, while the endogenous thrombin potential (ETP) represents the total amount of thrombin produced during the clotting process. ETP is the parameter most predictive of bleeding or thrombosis [16]. A shortened lag time and increased ETP/peak thrombin suggests a hypercoagulable status, while a prolonged lag time and reduction in ETP/peak thrombin suggests a hypocoagulable state. In this review, the applications and limitations of CAT in neonates are described (Table 1; Fig. 3).
The neonatal applications of CAT. CAT has been used to evaluate the differences in secondary haemostasis in preterm and term infants compared with adults and the relative haemostatic effect of platelets and extracellular vesicles in neonates. The use of CAT as a mechanism to test haemostatic therapies in a pre-clinical model and in infants at high risk of haemorrhage, particularly those undergoing cardiopulmonary bypass are also described. (Image created with BioRender.com)
Physiological haemostasis
Physiological coagulation is triggered by exposure of sub-endothelial tissues at the site of vascular injury [17]. The extrinsic pathway is activated by sub-endothelial tissue factor (TF) and circulating activated FVII, generating thrombin via the common pathway and activating the intrinsic pathway. Thrombin cleaves fibrinogen to fibrin, thus stabilising the clot. Platelets also become activated at the site of vascular injury through interactions with sub-endothelial tissues, von Willebrand factor, fibrinogen, and agonists such as thrombin. To limit coagulation to the site of injury, there are several inhibitory mechanisms, including tissue factor pathway inhibitor (TFPI), antithrombin, protein C pathway and fibrinolysis. Pathological changes to pro- or anti-coagulant activity can result in excessive bleeding or thrombosis [18].
Haemostasis in healthy term neonates
While FVIII and von Willebrand factor levels are usually within or above the healthy adult range at birth, levels of most other procoagulant factors are lower [2, 3]. Term infants do not display increased bleeding tendency despite these reduced levels. Levels of several anticoagulant factors (antithrombin, protein C, protein S) are also reduced [2, 3].
Six studies used CAT to characterise thrombin generation in platelet-poor plasma from term newborns compared with adults [19,20,21,22,23,24]. All demonstrated a significantly shortened lag time and time to peak in neonates. However, neonates had significantly reduced ETP and peak thrombin compared with adults, although one study showed no difference [22]. Similar findings were described in neonatal platelet-rich plasma [25].
It is hypothesised that this reduction in lag time, and time to peak in neonates (suggestive of a hypercoagulable state) is due to reduced TFPI, and that the reduction in ETP and peak thrombin (suggestive of a hypocoagulable state) is due to lower levels of pro-coagulant factors (particularly factor II) [23, 26], which itself might be further offset by a reduction in physiological anti-coagulant factors such as antithrombin [3].
Haemostasis in the preterm infant
Preterm infants are at high risk of haemorrhage and thrombosis [27, 28] and have reduced levels of procoagulant (FIX, FXI, FXII and fibrinogen) and anti-coagulant factors (antithrombin, protein C and protein S) compared with term neonates [2].
Three studies evaluated CAT in PPP from preterm compared with term infants. In preterm infants > 30 weeks gestation, ETP was higher than term controls [29]. Neary et al. demonstrated a significantly shorter lag time and time to peak in umbilical cord blood in preterm infants (24–30 weeks gestation), but found no difference in ETP or peak thrombin between groups [8].
Most recently, Tripodi et al. characterised thrombin generation in peripheral blood in very low birth weight (VLBW) infants < 1500 g. VLBW infants had higher ETP than term controls [9]. However, infants < 30 weeks gestation had significantly lower ETP than infants > 30 weeks (unfortunately, no comparison to term ETP was provided). There was no difference in ETP between small for gestational age (SGA) and appropriately grown infants. These findings in SGA infants replicate findings by Sokou et al. using thromboelastography (TEG), an alternative global coagulation assay [30].
Using thrombomodulin (TM), a key regulator of the protein C pathway, ETP-TM ratio was higher in preterm infants, suggesting a resistance to Protein C and thus a procoagulant imbalance in preterm plasma [9]. Interestingly, the presence of a procoagulant imbalance in preterm plasma may predispose to IVH, possibly due to an increased risk of venous infarction and venous haemorrhage. This hypothesis is supported by data describing increased IVH risk associated with hereditary thrombophilia [10]. Moreover, a study using TEG demonstrated a trend towards hypercoagulability in premature infants with IVH, compared to those without [31]. While these findings are not conclusive, they highlight the inability of standard clotting tests (PT/APTTs) to accurately reflect the true complexity of haemostatic balance in vivo.
Evaluating the effect of neonatal platelets
Although thrombocytopenia is common in sick neonates [32], platelet number is a poor predictor of severe haemorrhage [33]. In the PlaNeT 2 study, more liberal platelet transfusions were associated with a significantly higher incidence of mortality or severe haemorrhage, in thrombocytopenic infants [34]. The cause of this is not yet clear [35], although several differences exist between neonatal and adult platelets [4, 7]. While neonatal platelets are hyporesponsive to multiple agonists in vitro [4,5,6], the in vivo haemostatic consequences of this are poorly understood.
CAT in PRP is performed using a reagent which contains tissue factor only (without a source of exogenous phospholipids). This renders the assay dependent upon the phospholipid content of PRP.
Haidl et al. compared thrombin generation in PRP from term cord blood and adults [25]. In neonatal PRP, there were no differences in any thrombin generation parameters at platelet counts of 10,000/µL and 100,000/µL, suggesting that neonatal thrombin generation is not dependent on absolute platelet number. In contrast, thrombin generation in adult PRP is dependent on platelet count [15, 36]. CAT was evaluated in TFPI-depleted adult PPP, following the addition of high or low concentrations of TFPI, and varying concentrations of platelets [25]. Lower levels of TFPI (to represent neonatal plasma) were associated with lower platelet dependency of thrombin generation. Although endogenous TFPI activity levels in cord blood and adult samples were not described and would have been useful to confirm the hypothesis, reduced TFPI activity in neonates has been reported [26, 37].
The respective effects of neonatal and adult platelets on thrombin generation were evaluated by CAT, following the addition of platelets (neonatal/adult) to PPP (neonatal/adult) [38]. Newborn and adult platelets supported thrombin generation comparably. This suggests that CAT parameters were primarily determined by the plasma present (neonatal/adult). These results differ from a similar study using TEG, which found that the “transfusion” of neonatal platelets resulted in a shorter reaction time in both neonatal and adult blood, while the transfusion of adult platelets to cord blood resulted in a greater maximal amplitude and clot firmness, compared to neonatal platelets [39].
Schlagenhauf et al. demonstrated that upon stimulation, neonatal platelets release fewer inorganic polyphosphates, a pro-coagulant substance released from dense granules of activated platelets [22]. Using CAT, exogenous polyphosphates had a lower relative impact on thrombin generation parameters in neonatal PPP, but exerted their maximal effect at lower concentrations than in adults. Lower TFPI levels rendered neonates more sensitive to the effect of polyphosphate, while limiting the potential impact.
Different PRP preparation techniques were used in the platelet studies, which may explain some variability in the findings. Haidl et al. centrifuged whole blood at 200 × g for 10 min and diluted this with PPP to produce a specific platelet count (10, 50, 75 and 100 × 10^9/L) [25]. Peterson et al. centrifuged whole blood at 100 × g for 10 min, before diluting with PPP to achieve a standard platelet count of 50 × 10^9/L [40]. Bernhard et al. pelleted and washed platelets, before re-suspending them in PPP and adjusting “to similar counts” [38]. The International Society on Thrombosis and Haemostasis recommends centrifugation at 200 × g for 10 min with no brake to produce PRP [41]. These recommendations, to reduce red cell contamination and maintain platelet quiescence, derive from an adult study [42]. In CAT, PPP is often added to PRP to standardise platelet counts. The study by Haidl et al. suggests that in term neonates, PRP platelet counts do not influence thrombin generation parameters [25].
To date, no studies have evaluated the effect of premature platelets on neonatal haemostasis.
The effect of extracellular vesicles on haemostasis
Extracellular vesicles (EVs) are nanoparticles (ranging from 50 to 1000 nm) released from cells [43], surrounded by a lipid bi-layer. A majority (> 70%) of plasma EVs are derived from platelets [44], typically released upon their activation [45]. EVs may play a role in haemostasis, increasing the phospholipid surface for the enzymatic reactions of the coagulation cascade and potentially increasing the local concentration of TF present [46]. TF bearing EVs originate from many cells, including endothelial cells and monocytes [47, 48]. Several studies have demonstrated an increase in the number of platelet-derived EVs and procoagulant EV activity in neonates compared with adults [37, 49,50,51,52,53].
CAT was used to evaluate the procoagulant effect of EVs in term cord blood compared with adults [21]. CAT was performed using Thrombinoscope BV PPP reagent (tissue factor and phospholipid) and microparticle (MP) reagent (phospholipid only). The MP/PPP ratio was used to evaluate the relative effect of TF-EVs on thrombin generation. TF-EVs had a greater impact on thrombin generation in neonates than adults. This increased procoagulant EV activity supports the possible compensatory role of EVs in the neonatal haemostatic system.
CAT as a predictor of clinical bleeding in neonates
Standard clotting tests do not accurately predict the risk of bleeding [8, 10, 11]. Numerous studies have evaluated CAT as a predictor of bleeding in adults [54, 55], but few have in neonates. Peterson et al. found that CAT did not predict post-operative bleeding after cardiopulmonary bypass (CPB) [40]. Tripodi et al. found no difference in ETP measurements at birth, between VLBW infants that developed an IVH and those that did not [9]. Similarly, Neary et al. demonstrated no difference in any thrombin generation parameters between infants who developed a severe (or any) IVH and those that did not [56]. The current clinical application of CAT in neonates is limited by a lack of evidence to support CAT as a predictor of bleeding.
CAT to evaluate haemostatic therapies in neonates
Sick neonates frequently receive blood products and haemostatic drugs, but few randomised controlled trials have evaluated their use in this population [34, 57]. Neonatal doses are frequently extrapolated from adult regimens, and guidelines are often consensus agreements. PlaNeT 2 has raised awareness of the potential harms of blood products in neonates [34]. Haemostatic drugs must be evaluated in neonates, given the differences in neonatal factor levels [2, 3]. CAT has been used as a pre-clinical tool to evaluate the potential haemostatic effects of drugs in neonates.
Cvirn et al. evaluated the anti-coagulant effect of Melagatran, a direct thrombin inhibitor, in neonatal cord blood and adult PPP [20]. While a similar concentration of Melagatran was required to prolong the lag time and time to peak in both groups, both ETP and peak thrombin were suppressed by over 50% using a much lower drug concentration in cord blood plasma than that required to achieve the same effect in adult plasma. These distinct patterns of sensitivity to the same anticoagulant drug highlight the variability in endogenous haemostatic pathway activity which exists between neonatal and adult plasma, detectable by CAT.
CAT assessed the effect of ex vivo addition of NovoSeven® (recombinant factor VIIa) or three-factor prothrombin complex (3f-PCC) (containing FII, FIX, FX, and a small amount of FVII) to PPP of term infants post CPB [58]. While NovoSeven® reduced the lag time only, 3f-PCC also significantly increased peak thrombin and velocity index, above pre-CPB levels.
Franklin et al. studied the effect of two “four-factor prothrombin complex concentrates” (4f-PCCs), one which contained FVII and the other FVIIa, in PPP from term infants who had undergone CPB [19]. While both concentrations increased the peak thrombin and velocity index, only the preparation containing FVIIa reduced lag time to pre-CPB levels. The lower dose of both drugs tested was sufficient to enhance thrombin generation in neonates.
The effect of NovoSeven® in umbilical cord blood and adult PRP was investigated [25], due to the high incidence of reported thrombotic adverse events in the neonatal population [59]. NovoSeven® altered clot dynamics; it did not alter ETP in either group but shortened the lag time and reduced the peak height in both groups, most significantly in the neonatal group. The effect of rFVIIa did not appear to be platelet dependent in vitro. Moreover, the dose response to rFVIIa was comparable between the neonates and adult PRP.
CAT cannot replace trials to evaluate the clinical effects of these drugs, but it may provide some insights into the relative effects of haemostatic therapies in supporting normal coagulation, at least in vitro.
CAT in specific populations
Infants undergoing cardiopulmonary bypass
Infants who require neonatal surgical correction of cardiac malformations with CPB are at high risk of post-operative haemorrhage, due to a dilution of coagulation factors, exposure to heparin anti-coagulation and activation of blood cells as a result of interactions with extravascular tissue and artificial tubing [60]. Neonates typically receive blood products and pro-coagulant drugs to overcome these challenges.
Two studies evaluated thrombin generation in neonates pre- and post-CPB (following the reversal of heparin and the administration of blood products) [19, 58]. Both demonstrated a prolonged lag time post-CPB and an increase in peak thrombin compared with pre-CPB samples.
Peterson et al. evaluated CAT in PRP compared with other coagulation assays, assessing heparin reversal and rebound effect, in neonates undergoing CPB [40]. CAT results were compared with thrombin-initiated fibrin clot kinetics (TFCK). Peak thrombin inversely correlated with high TFCK ratios (blood samples with the highest heparin activity).
Factor VIII deficiency
Neonates with factor VIII deficiency can develop severe haemorrhage [61]. CAT evaluated the effect of factor VIII levels in neonates in cord blood PPP, with varying levels of factor VIII, anti-thrombin and TFPI [24].
Factor VIII depleted neonatal plasma showed a slight prolongation in lag time and time to peak, with no change in peak thrombin. In a neonate with confirmed factor VIII deficiency, the lag time and time to peak were slightly prolonged; however, the peak thrombin was reduced by 25%.
An increase in TFPI levels resulted in a prolonged lag time and time to peak, but had little effect on peak height or ETP. In contrast, an increase in the anti-thrombin level resulted in a large reduction in ETP (64%) and peak height (33%) but no change to the lag time. This study illustrated the mechanisms by which the inhibitory pathways impact on thrombin generation parameters in neonates.
Cholestatic liver disease
Preterm infants are at risk of cholestasis due to prolonged parenteral nutrition use. Cholestasis reduces vitamin K absorption, and thus, vitamin K–dependent coagulation factors. It was hypothesised that infants with intestinal failure associated liver disease may have impaired thrombin generation [62]. CAT was performed in PPP at birth and day 30 in the presence of thrombomodulin. In spite of prolonged standard clotting tests in the liver disease group, there was no difference in ETP between groups at either timepoint.
Strengths and limitations of CAT compared with alternative global coagulation assays
CAT is a useful tool to evaluate in detail the mechanisms of haemostasis in neonates, and to explore how these differ from adults. CAT evaluates for both hypo- and hypercoagulability [15] and has good intra- and inter-individual reliability [15]. Plasma CAT is a useful method of evaluating the function of specific plasma coagulation proteins.
CAT is only used as a research tool in neonates, not as a clinical assessment tool, and neonatal studies have derived from a small number of centres internationally. CAT requires the manual preparation of plasma and addition of reagents as described in Fig. 1. The reagents may be prepared on site or purchased from a manufacturer. Purchased reagents are costly, and CAT also requires specific instruments and software which would not be routinely available in clinical laboratories. There are challenges to the interchangeability of results between sites, particularly in relation to the pre-analytical steps and standardisation of reagents used, although studies have shown interchangeability between sites to be within acceptable ranges when standardised protocols are followed [63,64,65]. Currently, neonatal reference ranges or treatment algorithms that allow evidence-based prescribing of blood products do not exist for CAT. Moreover, CAT does not evaluate primary haemostasis or the effect of red or white cells, vascular endothelium or blood flow on secondary haemostasis [15].
Alternative global coagulation assays, such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM), use viscoelastic techniques to evaluate clot formation [66]. These may be useful in overcoming some limitations of CAT (Table 2). TEG/ROTEM evaluate the fibrinolytic system and are performed in whole blood, thus assessing the effect of all cellular blood components, requiring less time and skill to prepare, and reducing the risk of iatrogenic platelet activation. Standard CAT in duplicate requires up to 320 µL of plasma, while TEG requires only 340 µL of whole blood per analysis [67]. The blood volume required limits the use of CAT in neonates. Several CAT studies are performed in umbilical cord blood, given the larger volumes available. However, there is some evidence of a procoagulant imbalance in cord blood compared with neonatal blood, which may limit its applications [68]. The intra-assay reliability is acceptable in TEG, even in VLBW infants [67], and neonatal TEG reference ranges exist for both term and preterm infants [69, 70].
Conclusions
CAT studies have expanded our knowledge of crucial differences between neonatal and adult haemostasis and potential compensatory mechanisms in neonates. These present a narrative, that neonates, including preterm neonates, may have equivalent haemostatic potential or even be hypercoagulable compared with adults. This questions the practice of administering blood products and pro-coagulant drugs to non-bleeding neonates with deranged standard clotting tests.
Moving forward, CAT represents a useful research tool to evaluate haemostatic therapies and to expand our understanding of the intricacies of neonatal haemostasis. However, the role of CAT as a bedside clinical tool is limited, and this function may be better suited to whole blood assays such as TEG/ROTEM.
Abbreviations
- 3f-PCC:
-
Three-factor prothrombin complex concentrate
- 4f-PCC:
-
Four-factor prothrombin complex concentrate
- APTT:
-
Activated partial thromboplastin time
- CAT:
-
Calibrated automated thrombogram
- CPB:
-
Cardiopulmonary bypass
- ETP:
-
Endogenous thrombin potential
- EV:
-
Extracellular vesicle
- IVH:
-
Intraventricular haemorrhage
- MP:
-
Microparticle
- PPP:
-
Platelet poor plasma
- PRP:
-
Platelet-rich plasma
- PT:
-
Prothrombin time
- ROTEM:
-
Rotational thromboelastometry
- SGA:
-
Small for gestational age
- TEG:
-
Thromboelastography
- TF:
-
Tissue factor
- TFCK:
-
Thrombin initiated fibrin clot kinetics
- TFPI:
-
Tissue factor pathway inhibitor
- TM:
-
Thrombomodulin
- VLBW:
-
Very low birth weight
References
Ballabh P (2010) Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res 67(1):1–8. https://doi.org/10.1203/PDR.0b013e3181c1b176
Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Castle V, Powers P (1988) Development of the human coagulation system in the healthy premature infant. Blood 72(5):1651–1657
Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Powers P (1987) Development of the human coagulation system in the full-term infant. Blood 70(1):165–172
Sitaru AG, Holzhauer S, Speer CP, Singer D, Obergfell A, Walter U, Grossmann R (2005) Neonatal platelets from cord blood and peripheral blood. Platelets 16(3–4):203–210. https://doi.org/10.1080/09537100400016862
Rajasekhar D, Barnard MR, Bednarek FJ, Michelson AD (1997) Platelet hyporeactivity in very low birth weight neonates. Thromb Haemost 77(5):1002–1007
Grosshaupt B, Muntean W, Sedlmayr P (1997) Hyporeactivity of neonatal platelets is not caused by preactivation during birth. Eur J Pediatr 156(12):944–948. https://doi.org/10.1007/s004310050748
Ferrer-Marin F, Stanworth S, Josephson C, Sola-Visner M (2013) Distinct differences in platelet production and function between neonates and adults: implications for platelet transfusion practice. Transfusion 53(11):2814–2821. https://doi.org/10.1111/trf.12343
Neary E et al (2015) Coagulation indices in very preterm infants from cord blood and postnatal samples. J Thromb Haemost 13(11):2021–2030. https://doi.org/10.1111/jth.13130
Tripodi A et al (2020) Procoagulant imbalance in preterm neonates detected by thrombin generation procedures. Thromb Res 185:96–101. https://doi.org/10.1016/j.thromres.2019.11.013
Ramenghi LA, Fumagalli M, Groppo M, Consonni D, Gatti L, Bertazzi PA, Mannucci PM, Mosca F (2011) Germinal matrix hemorrhage: intraventricular hemorrhage in very-low-birth-weight infants. Stroke 42(7):1889–1893. https://doi.org/10.1161/STROKEAHA.110.590455
Christensen RD, Baer VL, Lambert DK, Henry E, Ilstrup SJ, Bennett ST (2014) Reference intervals for common coagulation tests of preterm infants (CME). Transfusion 54(3):627–632. https://doi.org/10.1111/trf.12322
Lancé MD (2015) A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thromb J 13:1–1. https://doi.org/10.1186/1477-9560-13-1
Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, Lecompte T, Beguin S (2002) The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb 32(5–6):249–253. https://doi.org/10.1159/000073575
Pal S, Curley A, Stanworth SJ (2015) Interpretation of clotting tests in the neonate. Arch Dis Child Fetal Neonatal Ed 100(3):F270–F274. https://doi.org/10.1136/archdischild-2014-306196
Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Béguin S (2003) Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb 33(1):4–15. https://doi.org/10.1159/000071636
Castoldi E, Rosing J (2011) Thrombin generation tests. Thromb Res 127(Suppl 3):S21–S25. https://doi.org/10.1016/S0049-3848(11)70007-X
Versteeg HH, Heemskerk JW, Levi M, Reitsma PH (2013) New fundamentals in hemostasis. Physiol Rev 93(1):327–358. https://doi.org/10.1152/physrev.00016.2011
Levi M, Opal SM (2006) Coagulation abnormalities in critically ill patients. Crit Care 10(4):222. https://doi.org/10.1186/cc4975
Franklin SW, Szlam F, Fernandez JD, Leong T, Tanaka KA, Guzzetta NA (2016) Optimizing thrombin generation with 4-factor prothrombin complex concentrates in neonatal plasma after cardiopulmonary bypass. Anesth Analg 122(4):935–942. https://doi.org/10.1213/ANE.0000000000001098
Cvirn G, Cimenti C, Kutschera J, Ferstl U, Wagner T, Muntean W, Jurgens G, Gallistl S, Koestenberger M (2007) Anticoagulant action of melagatran: a comparison between neonates and adults using calibrated automated thrombography (CAT). Eur J Pediatr 166(5):427–431. https://doi.org/10.1007/s00431-006-0253-6
Schweintzger S, Schlagenhauf A, Leschnik B, Rinner B, Bernhard H, Novak M, Muntean W (2011) Microparticles in newborn cord blood: slight elevation after normal delivery. Thromb Res 128(1):62–67. https://doi.org/10.1016/j.thromres.2011.01.013
Schlagenhauf A, Haidl H, Pohl S, Weiss EC, Leschnik B, Gallistl S, Muntean W (2017) Polyphosphate in neonates: less shedding from platelets and divergent prothrombotic capacity due to loweR TFPI levels. Front Physiol 8:586. https://doi.org/10.3389/fphys.2017.00586
Haidl H, Zohrer E, Pohl S, Leschnik B, Weiss EC, Gallistl S, Muntean W, Schlagenhauf A (2019) New insights into neonatal coagulation: normal clot formation despite lower intra-clot thrombin levels. Pediatr Res 86(6):719–724. https://doi.org/10.1038/s41390-019-0531-4
Fritsch P, Cvirn G, Cimenti C, Baier K, Gallistl S, Koestenberger M, Roschitz B, Leschnik B, Muntean W (2006) Thrombin generation in factor VIII-depleted neonatal plasma: nearly normal because of physiologically low antithrombin and tissue factor pathway inhibitor. J Thromb Haemost 4(5):1071–1077. https://doi.org/10.1111/j.1538-7836.2006.01947.x
Haidl H, Pohl S, Leschnik B, Gallistl S, Muntean W, Schlagenhauf A (2019) Neonatal thrombocytopenia: thrombin generation in presence of reduced platelet counts and effects of rFVIIa in cord blood. Sci Rep 9(1):8014. https://doi.org/10.1038/s41598-019-44199-y
Cvirn G, Gallistl S, Leschnik B, Muntean W (2003) Low tissue factor pathway inhibitor (TFPI) together with low antithrombin allows sufficient thrombin generation in neonates. J Thromb Haemost 1(2):263–268. https://doi.org/10.1046/j.1538-7836.2003.00081.x
Haley KM (2017) Neonatal venous thromboembolism. Front Pediatr 5:136–136. https://doi.org/10.3389/fped.2017.00136
Gleissner M, Jorch G, Avenarius S (2000) Risk factors for intraventricular hemorrhage in a birth cohort of 3721 premature infants. J Perinat Med 28(2):104–110. https://doi.org/10.1515/jpm.2000.013
Tripodi A, Ramenghi LA, Chantarangkul V, De Carli A, Clerici M, Groppo M, Mosca F, Mannucci PM (2008) Normal thrombin generation in neonates in spite of prolonged conventional coagulation tests. Haematologica 93(8):1256–1259. https://doi.org/10.3324/haematol.12566
Sokou R et al (2019) Thromboelastometry: studying hemostatic profile in small for gestational age neonates-a pilot observational study. Eur J Pediatr 178(4):551–557. https://doi.org/10.1007/s00431-019-03331-w
Radicioni M, Bruni A, Bini V, Villa A, Ferri C (2015) Thromboelastographic profiles of the premature infants with and without intracranial hemorrhage at birth: a pilot study. J Matern Fetal Neonatal Med 28(15):1779–1783. https://doi.org/10.3109/14767058.2014.968773
Sola-Visner M, Saxonhouse MA, Brown RE (2008) Neonatal thrombocytopenia: what we do and don’t know. Early Hum Dev 84(8):499–506. https://doi.org/10.1016/j.earlhumdev.2008.06.004
Stanworth SJ, Clarke P, Watts T, Ballard S, Choo L, Morris T, Murphy MF, Roberts I (2009) Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics 124(5):e826–e834. https://doi.org/10.1542/peds.2009-0332
Curley A et al (2019) Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med 380(3):242–251. https://doi.org/10.1056/NEJMoa1807320
Moore CM, Curley A (2019) Platelet transfusion thresholds in neonatal medicine. Early Human Dev 138:104845. https://doi.org/10.1016/j.earlhumdev.2019.104845
Siegemund T, Petros S, Siegemund A, Scholz U, Engelmann L (2003) Thrombin generation in severe haemophilia A and B: the endogenous thrombin potential in platelet-rich plasma. Thromb Haemost 90(5):781–786. https://doi.org/10.1160/TH03-01-0027
Uszyński M, Zekanowska E, Uszyński W, Kuczyński J, Zyliński A (2011) Microparticles (MPs), tissue factor (TF) and tissue factor inhibitor (TFPI) in cord blood plasma. A preliminary study and literature survey of procoagulant properties of MPs. Eur J Obstet Gynecol Reprod Biol 158(1):37–41. https://doi.org/10.1016/j.ejogrb.2011.04.026
Bernhard H, Rosenkranz A, Petritsch M, Kofeler H, Rehak T, Novak M, Muntean W (2009) Phospholipid content, expression and support of thrombin generation of neonatal platelets. Acta Paediatr 98(2):251–255. https://doi.org/10.1111/j.1651-2227.2008.01075.x
Ferrer-Marin F, Chavda C, Lampa M, Michelson AD, Frelinger AL 3rd, Sola-Visner M (2011) Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost 9(5):1020–1028. https://doi.org/10.1111/j.1538-7836.2011.04233.x
Peterson JA, Maroney SA, Zwifelhofer W, Wood JP, Yan K, Bercovitz RS, Woods RK, Mast AE (2018) Heparin-protamine balance after neonatal cardiopulmonary bypass surgery. J Thromb Haemost 16(10):1973–1983. https://doi.org/10.1111/jth.14245
Cattaneo M et al (2013) Recommendations for the standardization of light transmission aggregometry: a consensus of the Working Party from the Platelet Physiology Subcommittee of SSC/ISTH. J Thromb Haemost. https://doi.org/10.1111/jth.12231
Femia EA, Pugliano M, Podda G, Cattaneo M (2012) Comparison of different procedures to prepare platelet-rich plasma for studies of platelet aggregation by light transmission aggregometry. Platelets 23(1):7–10. https://doi.org/10.3109/09537104.2011.596592
Théry C et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750
Berckmans RJ, Nieuwland R, Böing AN, Romijn FP, Hack CE, Sturk A (2001) Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost 85(4):639–646
Warren BA, Vales O (1972) The release of vesicles from platelets following adhesion to vessel walls in vitro. Br J Exp Pathol 53(2):206–215
Owens AP 3rd, Mackman N (2011) Microparticles in hemostasis and thrombosis. Circ Res 108(10):1284–1297. https://doi.org/10.1161/circresaha.110.233056
Aharon A, Tamari T, Brenner B (2008) Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost 100(5):878–885. https://doi.org/10.1160/th07-11-0691
Holnthoner W et al (2017) Endothelial cell-derived extracellular vesicles size-dependently exert procoagulant activity detected by thromboelastometry. Sci Rep 7(1):3707. https://doi.org/10.1038/s41598-017-03159-0
Michelson AD, Rajasekhar D, Bednarek FJ, Barnard MR (2000) Platelet and platelet-derived microparticle surface factor V/Va binding in whole blood: differences between neonates and adults. Thromb Haemost 84(4):689–694
Schmugge M, Rand ML, Bang KW, Mody M, Dunn MS, Amankwah KS, Blanchette VS, Freedman J (2003) The relationship of von Willebrand factor binding to activated platelets from healthy neonates and adults. Pediatr Res 54(4):474–479. https://doi.org/10.1203/01.Pdr.0000081294.26060.4b
Wasiluk A, Mantur M, Szczepanski M, Matowicka-Karna J, Kemona H, Warda J (2008) Platelet-derived microparticles and platelet count in preterm newborns. Fetal Diagn Ther 23(2):149–152. https://doi.org/10.1159/000111597
Campello E et al (2015) Circulating microparticles in umbilical cord blood in normal pregnancy and pregnancy with preeclampsia. Thromb Res 136(2):427–431. https://doi.org/10.1016/j.thromres.2015.05.029
Korbal P, Slomka A, Sadowska-Krawczenko I, Zekanowska E (2017) Evaluation of tissue factor bearing microparticles in the cord blood of preterm and term newborns. Thromb Res 153:95–96. https://doi.org/10.1016/j.thromres.2017.02.017
Dargaud Y, Beguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, Hemker HC, Negrier C (2005) Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost 93(3):475–480. https://doi.org/10.1160/TH04-10-0706
Rugeri L, Beguin S, Hemker C, Bordet JC, Fleury R, Chatard B, Negrier C, Dargaud Y (2007) Thrombin-generating capacity in patients with von Willebrand’s disease. Haematologica 92(12):1639–1646. https://doi.org/10.3324/haematol.11460
Neary E (2016) To characterize standard laboratory coagulation parameters and plasma thrombin generation in very preterm infants and to investigate their relationship to clinical outcomes, in Department of Paediatrics. Royal College of Surgeons in Ireland: e-publications@rcsi
Andrew M et al (1993) A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr 123(2):285–291. https://doi.org/10.1016/s0022-3476(05)81705-6
Guzzetta NA, Szlam F, Kiser AS, Fernandez JD, Szlam AD, Leong T, Tanaka KA (2014) Augmentation of thrombin generation in neonates undergoing cardiopulmonary bypass. Br J Anaesth 112(2):319–327. https://doi.org/10.1093/bja/aet355
Witmer CM, Huang YS, Lynch K, Raffini LJ, Shah SS (2011) Off-label recombinant factor VIIa use and thrombosis in children: a multi-center cohort study. J Pediatr 158(5):820-825.e1. https://doi.org/10.1016/j.jpeds.2010.10.038
Arnold PD (2014) Coagulation and the surgical neonate. Pediatr Anesth 24(1):89–97. https://doi.org/10.1111/pan.12296
Ljung R, Lindgren AC, Petrini P, Tengborn L (1994) Normal vaginal delivery is to be recommended for haemophilia carrier gravidae. Acta Paediatr 83(6):609–611. https://doi.org/10.1111/j.1651-2227.1994.tb13090.x
Ghirardello S, Raffaeli G, Scalambrino E, Cortesi V, Roggero P, Peyvandi F, Mosca F, Tripodi A (2020) Thrombin generation in preterm newborns with intestinal failure-associated liver disease. Front Pediatr 8:510. https://doi.org/10.3389/fped.2020.00510
van Veen JJ, Gatt A, Makris M (2008) Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol 142(6):889–903. https://doi.org/10.1111/j.1365-2141.2008.07267.x
Tripodi A (2016) Thrombin generation assay and its application in the clinical laboratory. Clin Chem 62(5):699–707. https://doi.org/10.1373/clinchem.2015.248625
Ljungkvist M et al (2019) Evaluation of a standardized protocol for thrombin generation using the calibrated automated thrombogram: A Nordic study. Haemophilia 25(2):334–342. https://doi.org/10.1111/hae.13640
Whiting D, DiNardo JA (2014) TEG and ROTEM: technology and clinical applications. Am J Hematol 89(2):228–232. https://doi.org/10.1002/ajh.23599
Ghirardello S, Raffaeli G, Scalambrino E, Chantarangkul V, Cavallaro G, Artoni A, Mosca F, Tripodi A (2018) The intra-assay reproducibility of thromboelastography in very low birth weight infants. Early Hum Dev 127:48–52. https://doi.org/10.1016/j.earlhumdev.2018.10.004
Raffaeli G et al (2020) Is placental blood a reliable source for the evaluation of neonatal hemostasis at birth? Transfusion 60(5):1069–1077. https://doi.org/10.1111/trf.15785
Motta M, Guaragni B, Pezzotti E, Rodriguez-Perez C, Chirico G (2017) Reference intervals of citrated-native whole blood thromboelastography in premature neonates. Early Hum Dev 115:60–63. https://doi.org/10.1016/j.earlhumdev.2017.09.014
Sewell EK, Forman KR, Wong EC, Gallagher M, Luban NL, Massaro AN (2017) Thromboelastography in term neonates: an alternative approach to evaluating coagulopathy. Arch Dis Child Fetal Neonatal Ed 102(1):F79-f84. https://doi.org/10.1136/archdischild-2016-310545
Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, Maybury H, Collins PW, Laffan M (2018) The use of viscoelastic haemostatic assays in the management of major bleeding. Br J Haematol 182(6):789–806. https://doi.org/10.1111/bjh.15524
Funding
Open Access funding provided by the IReL Consortium. CAM is funded by a grant from the National Children’s Research Centre, Dublin, Ireland.
Author information
Authors and Affiliations
Contributions
CAM designed and drafted this review. EN, DOR, SC, AE, FN, PBM, NM and BK contributed to the design, edited this review, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murphy, C.A., Neary, E., O’Reilly, D.P. et al. The role of the calibrated automated thrombogram in neonates: describing mechanisms of neonatal haemostasis and evaluating haemostatic drugs. Eur J Pediatr 181, 23–33 (2022). https://doi.org/10.1007/s00431-021-04196-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04196-8