Abstract
Viral lower respiratory tract infection (VLRTI) is the most common cause of hospital admission among small children in high-income countries. Guidelines to identify children in need of admission are lacking in the literature. In December 2012, our hospital introduced strict guidelines for admission. This study aims to retrospectively evaluate the safety and efficacy of the guidelines. We performed a single-center retrospective administrative database search and medical record review. ICD-10 codes identified children < 24 months assessed at the emergency department for VLRTI for a 10-year period. To identify adverse events related to admission guidelines implementation, we reviewed patient records for all those discharged on primary contact followed by readmission within 14 days. During the study period, 3227 children younger than 24 months old were assessed in the ED for VLRTI. The proportion of severe adverse events among children who were discharged on their initial emergency department contact was low both before (0.3%) and after the intervention (0.5%) (p=1.0). Admission rates before vs. after the intervention were for previously healthy children > 90 days 65.3% vs. 53.3% (p<0.001); for healthy children ≤ 90 days 85% vs. 68% (p<0.001); and for high-risk comorbidities 74% vs. 71% (p=0.5).
Conclusion: After implementation of admission guidelines for VLRTI, there were few adverse events and a significant reduction in admissions to the hospital from the emergency department. Our admission guidelines may be a safe and helpful tool in the assessment of children with VLRTI.
What is Known: • Viral lower respiratory tract infection, including bronchiolitis, is the most common cause of hospitalization for young children in the developed world. Treatment is mainly supportive, and hospitalization should be limited to the cases in need of therapeutic intervention. • Many countries have guidelines for the management of the disease, but the decision on whom to admit for inpatient treatment is often subjective and may vary even between physicians in the same hospital. | |
What is New: • Implementation of admission criteria for viral lower respiratory tract infection may reduce the rate of hospital admissions without increasing adverse events. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Viral lower respiratory tract infection (VLRTI) including bronchiolitis and viral pneumonia is the leading cause of hospitalization in children under 24 months of age in high-income countries [1,2,3,4,5,6]. It is a clinical diagnosis, with the main clinical features being coryza, poor feeding, difficulty breathing, cough, wheeze, and crepitations on auscultation [2, 3, 7]. Management of VLRTI is primarily supportive, with no effective therapies available [1, 2, 8, 9]. There is iatrogenic risk connected to hospitalization, due to medical management errors [10] and nosocomial infections [11, 12]. In addition, one study indicated that excessive handling of children hospitalized for VLTRI can increase both the length of stay and the need for supportive care [13]. Treatment should therefore promote minimal handling, and hospitalization should be limited to cases in need of therapeutic intervention.
Despite the prevalence of the disease, the emergency department (ED) decision to hospitalize is often subjective and may therefore vary between physicians in the same hospital [14]. While many countries have guidelines for the management of VLRTI [1, 15,16,17], there are few guidelines for when hospital admission is necessary [18].
Several studies have developed prognostic criteria for patients with bronchiolitis, indicating which patients should be admitted [3, 15, 19,20,21,22,23,24,25] and leading to clinical risk score models that have been tested retrospectively for accuracy [26,27,28]. However, the models usually assume that the ED decision to admit the patient was always appropriate, using this as the outcome measure. Inter-physician variability may thus limit the usefulness of such models. Lou et al. developed and studied an operational definition of appropriate hospital admission for bronchiolitis, which may be more objective: ≥ 6-h exposure to major medical interventions defined a necessary hospital admission; return to the ED within 12 h followed by hospitalization defined an unsafe ED discharge [29].
Studying patient data in our hospital, we found that many infants hospitalized with bronchiolitis did not receive any supportive therapy but were admitted for observation only. We saw a potential to improve our practice by the development of guidelines for patient admission.
The primary aim of this study was to evaluate the safety of the guidelines in previously healthy children aged > 90 days to < 24 months presenting with VLRTI. Secondary aims were to (i) assess safety in children aged ≤ 90 days, or with medical conditions predisposing to more severe VLRTI, and (ii) determine the guidelines’ efficacy to change hospital admission rates, return rates, or use of major medical interventions.
Materials and methods
This study is a retrospective analysis of pediatric admissions and re-admissions for VLRTI before and after the implementation of strict admission guidelines.
Admission criteria were based on both literature review and clinical information from a prospective study conducted on children assessed for viral airway infection in our ED [3, 30, 31]. Before implementation, the guidelines were discussed with the staff in both the ED and the inpatient department (IPD), including attending physicians and nurses.
In December 2012, these guidelines were implemented in the pediatric ED at Akershus University Hospital, with strict and precise criteria for admission to the IPD.
The admission guidelines with modifying risk factors are presented in Fig. 1 and apply to children less than 2 years of age with VLRTI with or without pneumonia, including children with a first episode of wheeze. The criteria do not apply to children with suspicion of bacterial infection or asthma.
To avoid short-term improvements in clinical parameters that might affect the decision to admit or send home, ED staff were discouraged from giving nebulized saline or bronchodilators to children awaiting a decision on admission.
As part of the intervention, parents of any child not admitted to the in-patient department were educated in management of their sick child before leaving the hospital. All parents were provided with a manual nasal aspirator (vacuum provided by oral suctioning) and trained in nasal suctioning of the child by the following procedure: (i) saline drops in each nostril 1 to 2 min before suctioning; (ii) gentle suctioning from the anterior nares; (iii) deep nasal suctioning discouraged; and (iv) procedure repeated as needed, ideally before feeding, maximum 8 times daily. Parents were encouraged to apply oxymetazoline drops up to three times daily. Parents were not routinely provided with nebulizers or similar devices for inhalation of saline or bronchodilators.
Admission criteria were implemented from December 1, 2012–February 1, 2013. This period is defined as the intervention, and patients admitted during this period were excluded from the study, to acknowledge that not all medical staff had become familiar with the new procedures. January 1, 2009–November 30, 2012, was defined as pre-intervention. February 2, 2013–December 31, 2019, was defined as post-intervention. During the intervention, authors CI and HOF conducted a 2-month period of intensive education of junior and senior doctors and nurses on the ED and demanded details of how guidelines were applied for each patient admitted from the ED. Guidelines were published on posters and in pocket format and made easily available to all staff and presented regularly during subsequent years as a part of the departmental teaching program. Author CI monitored patients who returned to the ED during implementation for serious adverse events.
Concurrently with admission guidelines implementation, we introduced guidelines for treatment and discharge of children with VLRTI. Treatment guidelines included the following: (i) promotion of minimal handling; (ii) restriction of supplementary oxygen to children with SpO2 < 92% for more than 2 h; (iii) children with SpO2 < 88% or cyanosis were given supplemental oxygen immediately; (iv) bronchodilators were discouraged; (v) nebulized saline was given on demand, rather than by fixed-schedule [13]; and (vi) non-invasive respiratory support was reserved for children with severe respiratory distress. Discharge criteria were as follows: (i) no need for supplemental oxygen the last 6 h; (ii) not clinically dehydrated; (iii) adequate fluid intake and urine production; (iv) parents have been trained in nasal suctioning as described above; and (v) no expectation of deterioration in condition, in particular if still early during the illness. Signs of respiratory distress alone were not considered a reason for continued admission if all other parameters were adequate.
Hospital setting
Akershus University Hospital serves a child population of approximately 125,000 children, of whom around 12,000 are younger than 2 years of age. The pediatric ED is a secondary health-care center, and any visit requires referral from a general practitioner or primary health-care emergency ward. The Norwegian public health-care system is organized in health districts, with clearly defined hospital catchment areas. There are no private hospitals providing emergency care for children. This ensures that unless patients travel to another part of the country during their illness, (i) patients living in the hospital’s catchment area are not referred to another hospital for emergency care and (ii) any child would return to the same ED if in need of a second visit. In addition, comorbidities associated with severe VLRTI would in almost all cases be known to the hospital prior to admission with VLRTI.
Patient identification and characteristics
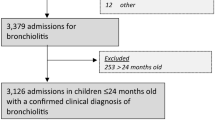
Data collection was conducted in September 2019. The data capture group at Akershus University Hospital accessed the hospital’s patient database, identifying children aged ≤ 24 months with an episode of care for VLRTI, defined by ICD-10 discharge codes (Online Resource 1), between January 2008 and August 2019.
For each patient, we collected background data (sex, age, comorbidities) and contact-specific data (length of stay, hospital readmission within 14 days of the primary contact). ICD-10 codes from any previous hospital contacts identified comorbidities (Online Resource 2).
To assess guidelines safety, (i) author LH reviewed electronic health records of all children admitted to the IPD within 14 days of discharge from the ED or IPD, determining if the readmission was due to the same episode of VLRTI. Adverse events and serious adverse events at the time of return were identified. Adverse events were defined as a patient returning in need of oxygen supplement or nasogastric fluid supplements administered within short time after arrival. Serious adverse events were defined as any child immediately given either intravenous fluids or respiratory support (high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), or bi-phasic positive airway pressure (BiPAP)) on readmission. (ii) We calculated readmission rates within 12 h, considering this as inappropriate discharge [27]. (iii) We compared overall return rates before and after the intervention.
To evaluate criteria efficacy, (i) we compared the overall rate of admission to the IPD on initial contact and for patients who were reassessed on the ED for the same episode of VLRTI. (ii) Author LH reviewed health records for major medical interventions (MMIs) in a subset of 160 randomly selected patients admitted to the IPD: 80 before and 80 after the intervention. MMI was defined as supplementary oxygen, rehydration (intravenous or by tube feeding), or any form of invasive or non-invasive respiratory support.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 25 and Stata version 16. Significance levels were 2-sided and set at p<0.05. Proportions were analyzed with Fischer’s exact test. Continuous data was analyzed using independent samples median test. For patients discharged from the ED on their initial visit, recontacts were plotted as Kaplan-Meier estimates and analyzed using log-rank test. Patients returning to the ED for reasons other than VLRTI were censored. All patients were censored at 360 h after the initial contact. Population-adjusted means of ED visits and IPD admissions were calculated by season (July 1, 2008, to June 30, 2009, etc.). As the number of observations was small, Mann-Whitney U test was used for comparison. Interrupted time series analysis was performed on admission rates, using segmented regression analysis. The first half of 2008 was an extreme outlier regarding the admission rate for VLRTI. The hospital moved to another location September 2008, possibly altering the hospital database. We were not able to identify or adjust for this; therefore admissions during the first half of 2008 were removed from population-based estimates, and admissions from the whole of 2008 removed from detailed analysis.
Ethics
The purpose of this study was quality improvement. The hospital’s data protection officer approved the study without need for informed consent or approval from the regional ethics committee.
Results
From January 1, 2009, to August 21, 2019, 3416 children under 2 years of age received a discharge diagnosis of VLRTI at Akershus University Hospital. The period from December 1, 2012, to February 1, 2013, was considered the intervention period during which guidelines were implemented. During the implementation period, 189 patients were treated and therefore excluded, leaving a total of 3227 children for analysis, 1136 before the intervention and 2091 after. Of these, the number of previously healthy children before the intervention was 753 (66%) and 1236 (60%) after the intervention. Baseline characteristics of the study population are listed in Table 1.
Adverse events
Eighteen children discharged from the ED after initial assessment experienced an adverse event, with similar proportions before and after the intervention, as described in Table 2. Among previously healthy children older than 90 days, non-severe adverse events occurred in 2 (0.8%) children before and 2 (0.3%) after the intervention (p= 0.6%), and serious adverse events in 0 (0.0%) cases before and 2 (0.3%) after the intervention (p=1.0). There were similar results for children ≤ 90 days and those with comorbidities.
One life-threatening event occurred. The patient had recovered from bronchiolitis and was discharged from the IPD. Seven days later, the child was readmitted for a post-viral cardiac complication, requiring immediate intensive care treatment. There were no mortalities among readmitted patients.
For children not admitted on their initial ED contact, the total number of recontacts within 12 h was 28 (2.4%), of whom, 14 (1.2%) were then admitted. There was no significant difference in recontacts after guideline implementation (Table 2).
Admission rates
The overall rate of admissions from the emergency department (ED) to the inpatient department (IPD) was 804/1136 (70.8%) before the intervention and 1244/2091 (59.5%) after the intervention (p<0.001). For previously healthy children > 90 days of age, the admission rate was 492/753 (65.3%) before the intervention and 673/1263 (53.3%) after the intervention (p<0.001). Table 2 shows admission rates (ED to IPD) on the initial hospital contact and on subsequent contacts, for the total group of children as well as for subgroups.
To test if the change in admission rates was temporary, interrupted time series analysis was performed (Online Resource 3). For previously healthy children > 90 days of age, the intervention gave an odds ratio for admission of 0.81 (CI 0.69–0.95). Considering all children, the intervention gave an odds ratio for admission of 0.88 (CI 0.78–0.99). After the intervention, the effect of time on the admission rate was not significant (previously healthy children > 90 days of age, odds ratio 1.00 (CI 0.99–1.01); all children, odds ratio 0.99 (CI 0.992–1.00)) indicating that the reduction in admission rate was consistent over time.
Crude rates of ED contacts and IPD admissions are presented in Table 2.
Proportion of recontact
A total of 1179 children were discharged from the ED on their initial contact, without admission to the IPD. Proportions of recontact within 14 days (336 h) for VLRTI and leading to hospitalization were plotted as Kaplan-Meier estimates. Children discharged from the ED on their initial contact had significantly less subsequent ED visits leading to hospitalization after the intervention (p=0.027). Subgroup analysis showed a similar reduction for previously healthy children > 90 days old (p=0.009) and for children with comorbidities (p=0.006). In previously healthy children ≤ 90 days old, we found no significant change (Fig. 2).
Time to readmission for children not admitted on first ED assessment. Kaplan-Meier plots of children who were not admitted to the ID on their initial contact, comparing the proportion managed at home before vs. after the intervention. Plots are for all children (a) and subgroups of children (b, c, d).
Major medical interventions
Children who had their medical records reviewed for major medical interventions had no significant differences in sex, age, or comorbidity before vs. after intervention (Table 1). In admitted, previously healthy children > 90 days, MMI use increased from 22 of 52 (42%) reviewed cases before to 33 of 43 (76%) reviewed cases after guidelines implementation (p=0.001) (Table 2).
Discussion
In this retrospective single-center study, we assessed the value of standardized admission criteria for children under 24 months of age with VLRTI, finding that adverse events did not increase while admission rates decreased. The overall incidence of adverse events in children with VLRTI, who were discharged home after ED assessment, was low both in the total study population and in the primary target group of this study, previously healthy children aged > 90 days. After guidelines implementation, parents were advised how to manage mild respiratory distress, primarily regular removal of nasal secretions by suctioning and adequate intake of fluids. There were no mortalities among patients returning to the hospital. There were no differences in adverse events or readmission within 12 h (defined as unsafe by Luo et al.[27]) after guidelines implementation.
Prior to the analysis, we suspected that our strict guidelines for hospitalization would reduce hospital admissions but increase recontacts and later hospital admissions. Our findings indicate the contrary that the implementation reduced not only IPD admissions on initial contact but also subsequent contacts to the ED. In adult patients, educational sessions are found to effectively improve self-management skills and reduce hospital readmissions [32,33,34]. The education of parents in nasal suctioning and general management of their sick child, including signs of clinical deterioration, may have increased parental confidence in managing the child at home. It is not possible to differentiate between the specific effect of the guidelines and that of parental education since they were implemented together, but it is likely that more confident parents did play an important role in reducing readmissions.
Guidelines implementation and adherence are often difficult to achieve. We resolved this by an intensive individualized education program and assessment of guidelines adherence in admitted children. Education of the doctors and nurses in the ED and increased focus on VLRTI in general may also have led to more homogeneous care provided on the initial visit, resulting in not only more appropriate hospitalizations but also more appropriate discharges from the initial ED visit. Standardizing patient care can have strong effects on admission parameters. One study found differences in LOS and hospital costs correlated to differences in care practices between hospitals [35]. Another study found reduction in LOS after introduction of discharge criteria [36].
Our guidelines had a positive impact on the overall rate of admission from the ED to the IPD, both on initial visit to the ED and on subsequent visits related to the same illness. As part of the guidelines, a target SpO2 level was introduced. Cunningham et al. found that acceptance of lower targeted SpO2 led to lower admission rates, and patients could be managed on full feeds sooner and had fewer readmissions to hospital [37].
In addition to fewer admissions to the IPD after guidelines introduction, we saw an increased use of major medical interventions among the subset of patients who had their medical records reviewed. Other studies support the use of MMI as a sign of more severe disease, and an increased proportion of patients receiving MMI indicates more appropriate hospitalizations [3, 27, 38]. A reduction in unnecessary hospital admission will not only reduce medical costs but also the potential iatrogenic risk associated with hospitalization of small children with VLRTI [10, 39,40,41].
The study is limited by its retrospective design, with few clinical variables available, and results may be influenced by unmeasured confounders. In particular, caution should be taken in interpreting results for children with comorbidities predisposing for severe disease, as this is a heterogeneous group with varying degrees of disease severity and often multiple factors that may interact [2, 8, 42, 43]. Studies assessing risk factors for major medical interventions in children with VLRTI often exclude high-risk groups from the analysis [24, 38, 44]. Doctors appointed after the intervention and with experience from other hospitals may make different decisions in the ED [14, 45], a factor that is difficult to account for. However, the reduction in admission rates remained stable over time (ESM 3), suggesting that new doctors either adopted the guidelines or else had a similar practice already. The hospital database did not systematically record parameters such as virology results, frequency and number of treatments given, duration of oxygen therapy, transcutaneous saturation oxygenation (SpO2) in ambient air, or number of daily feeds. A prospective data collection design, including qualitative assessment of junior doctors’ and nurses’ management practices and experiences, might have given more insight. We cannot exclude that some patients visited other parts of the country during their illness. However, this is unlikely to represent a significant number. Given the structure of the Norwegian health-care system and the number of patients assessed, we consider it unlikely that such situations will affect our results.
We conducted a single-center study, comparing outcome before versus after implementation of admission guidelines. Childhood VLRTI has considerable variability from year to year, with inter-seasonal differences in disease severity [46,47,48,49,50]. The guidelines were not intended for patients in whom the doctor suspected asthma, and they did not specifically define VLRTI or asthma. We cannot document why treating physicians diagnosed VLRTI instead of acute asthma, but recommendations at our site state that asthma may be considered from about 12 months of age in children with recurrent wheeze, especially if other atopic diseases or a family history of atopy. Since diagnostic decisions were not the focus of the guidelines or this study, we do not believe that occurrences of misdiagnosis will bias our results. However, differences in assessment and diagnosis of VLRTI between our site and other hospitals may affect replicability [14, 45].
A prospective study design with randomization of different hospitals to either guidelines or standard practice may have better assessed the efficacy of the guidelines, as this would control for differences in disease severity between different seasons and for differences in practice between clinical units.
Data from 2008 was omitted because of significantly higher admission rates compared to the other pre-intervention years, possibly caused by coding issues in the hospital database after moving site. This may introduce bias. However, since admission rates were higher during 2008, including this data would increase the strength of our findings. We therefore consider that this omission does not affect our conclusions.
The total number of adverse events was small, meaning the study did not have enough power to determine whether the guidelines significantly changed the occurrence of adverse events. However, the low number of adverse events indicates that the management of VLRTI among children < 2 years of age was safe, both before and after the introduction of admission guidelines.
In summary, we find that the implementation of our admission guidelines reduced both the admission rate and the return rate of previously healthy children both under and over 90 days of age with VLRTI. There were few adverse events.
Data availability
Not applicable.
Code availability
Not applicable
Change history
10 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00431-022-04780-6
Abbreviations
- BiPAP:
-
Bi-phasic positive airways pressure
- CPAP:
-
Continuous positive airway pressure
- ED:
-
Emergency department
- HFNC:
-
High-flow nasal cannula
- IPD:
-
Inpatient department
- MMI:
-
Major medical intervention
- VLRTI:
-
Viral lower respiratory tract infection
References
Florin TA, Plint AC, Zorc JJ (2017) Viral bronchiolitis. Lancet 389(10065):211–224. https://doi.org/10.1016/S0140-6736(16)30951-5
Meissner HC (2016) Viral bronchiolitis in children. N Engl J Med 374(1):62–72. https://doi.org/10.1056/NEJMra1413456
Parker MJ, Allen U, Stephens D, Lalani A, Schuh S (2009) Predictors of major intervention in infants with bronchiolitis. Pediatr Pulmonol 44(4):358–363. https://doi.org/10.1002/ppul.21010
Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr (2013) Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics 132(1):28–36. https://doi.org/10.1542/peds.2012-3877
Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, Alassani I, Ali A, Antonio M, Awasthi S, Awori JO, Azziz-Baumgartner E, Baggett HC, Baillie VL, Balmaseda A, Barahona A, Basnet S, Bassat Q, Basualdo W, Bigogo G, Bont L, Breiman RF, Brooks WA, Broor S, Bruce N, Bruden D, Buchy P, Campbell S, Carosone-Link P, Chadha M, Chipeta J, Chou M, Clara W, Cohen C, de Cuellar E, Dang DA, Dash-Yandag B, Deloria-Knoll M, Dherani M, Eap T, Ebruke BE, Echavarria M, de Freitas Lazaro Emediato CC, Fasce RA, Feikin DR, Feng L, Gentile A, Gordon A, Goswami D, Goyet S, Groome M, Halasa N, Hirve S, Homaira N, Howie SRC, Jara J, Jroundi I, Kartasasmita CB, Khuri-Bulos N, Kotloff KL, Krishnan A, Libster R, Lopez O, Lucero MG, Lucion F, Lupisan SP, Marcone DN, McCracken JP, Mejia M, Moisi JC, Montgomery JM, Moore DP, Moraleda C, Moyes J, Munywoki P, Mutyara K, Nicol MP, Nokes DJ, Nymadawa P, da Costa Oliveira MT, Oshitani H, Pandey N, Paranhos-Baccala G, Phillips LN, Picot VS, Rahman M, Rakoto-Andrianarivelo M, Rasmussen ZA, Rath BA, Robinson A, Romero C, Russomando G, Salimi V, Sawatwong P, Scheltema N, Schweiger B, Scott JAG, Seidenberg P, Shen K, Singleton R, Sotomayor V, Strand TA, Sutanto A, Sylla M, Tapia MD, Thamthitiwat S, Thomas ED, Tokarz R, Turner C, Venter M, Waicharoen S, Wang J, Watthanaworawit W, Yoshida LM, Yu H, Zar HJ, Campbell H, Nair H, Network RSVGE (2017) Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390(10098):946–958. https://doi.org/10.1016/S0140-6736(17)30938-8
Collaborators GL (2017) Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17(11):1133–1161. https://doi.org/10.1016/S1473-3099(17)30396-1
Hall CB, Simoes EA, Anderson LJ (2013) Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol 372:39–57. https://doi.org/10.1007/978-3-642-38919-1_2
Kirolos A, Manti S, Blacow R, Tse G, Wilson T, Lister M, Cunningham S, Campbell A, Nair H, Reeves RM, Fernandes RM, Campbell H, Investigators R (2019) A systematic review of clinical practice guidelines for the diagnosis and management of bronchiolitis. J Infect Dis 222:S672–S679. https://doi.org/10.1093/infdis/jiz240
Midulla F, Petrarca L, Frassanito A, Di Mattia G, Zicari AM, Nenna R (2018) Bronchiolitis clinics and medical treatment. Minerva Pediatr 70(6):600–611. https://doi.org/10.23736/S0026-4946.18.05334-3
McBride SC, Chiang VW, Goldmann DA, Landrigan CP (2005) Preventable adverse events in infants hospitalized with bronchiolitis. Pediatrics 116(3):603–608. https://doi.org/10.1542/peds.2004-2387
Macartney KK, Gorelick MH, Manning ML, Hodinka RL, Bell LM (2000) Nosocomial respiratory syncytial virus infections: the cost-effectiveness and cost-benefit of infection control. Pediatrics 106(3):520–526. https://doi.org/10.1542/peds.106.3.520
Hall CB (2000) Nosocomial respiratory syncytial virus infections: the "Cold War" has not ended. Clin Infect Dis 31(2):590–596. https://doi.org/10.1086/313960
Skjerven HO, Hunderi JO, Brugmann-Pieper SK, Brun AC, Engen H, Eskedal L, Haavaldsen M, Kvenshagen B, Lunde J, Rolfsjord LB, Siva C, Vikin T, Mowinckel P, Carlsen KH, Lodrup Carlsen KC (2013) Racemic adrenaline and inhalation strategies in acute bronchiolitis. N Engl J Med 368(24):2286–2293. https://doi.org/10.1056/NEJMoa1301839
Brand PL, Vaessen-Verberne AA (2000) Differences in management of bronchiolitis between hospitals in The Netherlands. Dutch Paediatric Respiratory Society. Eur J Pediatr 159(5):343–347
Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa NF, Mendonca EA, Phelan KJ, Zorc JJ, Stanko-Lopp D, Brown MA, Nathanson I, Rosenblum E, Sayles S 3rd, Hernandez-Cancio S, American Academy of P (2014) Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 134(5):e1474–e1502. https://doi.org/10.1542/peds.2014-2742
O'Brien S, Wilson S, Gill FJ, Cotterell E, Borland ML, Oakley E, Dalziel SR, Paediatric Research in Emergency Departments International Collaborative network A (2018) The management of children with bronchiolitis in the Australasian hospital setting: development of a clinical practice guideline. BMC Med Res Methodol 18(1):22. https://doi.org/10.1186/s12874-018-0478-x
Carande EJ, Galiza EP, Nickless A, Pollard AJ, Drysdale SB (2018) Viral bronchiolitis management in hospitals in the UK. J Clin Virol 104:29–33. https://doi.org/10.1016/j.jcv.2018.04.010
Luo G, Nkoy FL, Gesteland PH, Glasgow TS, Stone BL (2014) A systematic review of predictive modeling for bronchiolitis. Int J Med Inform 83(10):691–714. https://doi.org/10.1016/j.ijmedinf.2014.07.005
Mansbach JM, Clark S, Christopher NC, LoVecchio F, Kunz S, Acholonu U, Camargo CA Jr (2008) Prospective multicenter study of bronchiolitis: predicting safe discharges from the emergency department. Pediatrics 121(4):680–688. https://doi.org/10.1542/peds.2007-1418
Voets S, van Berlaer G, Hachimi-Idrissi S (2006) Clinical predictors of the severity of bronchiolitis. Eur J Emerg Med 13(3):134–138. https://doi.org/10.1097/01.mej.0000206194.85072.33
Walsh P, Rothenberg SJ, O'Doherty S, Hoey H, Healy R (2004) A validated clinical model to predict the need for admission and length of stay in children with acute bronchiolitis. Eur J Emerg Med 11(5):265–272
Destino L, Weisgerber MC, Soung P, Bakalarski D, Yan K, Rehborg R, Wagner DR, Gorelick MH, Simpson P (2012) Validity of respiratory scores in bronchiolitis. Hosp Pediatr 2(4):202–209
Corneli HM, Zorc JJ, Holubkov R, Bregstein JS, Brown KM, Mahajan P, Kuppermann N, Bronchiolitis Study Group for the Pediatric Emergency Care Applied Research N (2012) Bronchiolitis: clinical characteristics associated with hospitalization and length of stay. Pediatr Emerg Care 28(2):99–103. https://doi.org/10.1097/PEC.0b013e3182440b9b
Schuh S, Kwong JC, Holder L, Graves E, Macdonald EM, Finkelstein Y (2018) Predictors of critical care and mortality in bronchiolitis after emergency department discharge. J Pediatr 199:217–222 e211. https://doi.org/10.1016/j.jpeds.2018.04.010
Yusuf S, Caviness AC, Adekunle-Ojo AO (2012) Risk factors for admission in children with bronchiolitis from pediatric emergency department observation unit. Pediatr Emerg Care 28(11):1132–1135. https://doi.org/10.1097/PEC.0b013e31827132ff
Marlais M, Evans J, Abrahamson E (2011) Clinical predictors of admission in infants with acute bronchiolitis. Arch Dis Child 96(7):648–652. https://doi.org/10.1136/adc.2010.201079
Luo G, Johnson MD, Nkoy FL, He S, Stone BL (2018) Appropriateness of hospital admission for emergency department patients with bronchiolitis: secondary analysis. JMIR Med Inform 6(4):e10498. https://doi.org/10.2196/10498
Rodriguez-Martinez CE, Sossa-Briceno MP, Nino G (2018) Systematic review of instruments aimed at evaluating the severity of bronchiolitis. Paediatr Respir Rev 25:43–57. https://doi.org/10.1016/j.prrv.2016.12.006
Luo G, Stone BL, Johnson MD, Nkoy FL (2016) Predicting appropriate admission of bronchiolitis patients in the emergency department: rationale and methods. JMIR Res Protoc 5(1):e41. https://doi.org/10.2196/resprot.5155
Inchley CS, Sonerud T, Fjaerli HO, Nakstad B (2015) Nasal mucosal microRNA expression in children with respiratory syncytial virus infection. BMC Infect Dis 15:150. https://doi.org/10.1186/s12879-015-0878-z
Solevag AL, Eggen EH, Schroder J, Nakstad B (2013) Use of a modified pediatric early warning score in a department of pediatric and adolescent medicine. PLoS One 8(8):e72534. https://doi.org/10.1371/journal.pone.0072534
Rice H, Say R, Betihavas V (2018) The effect of nurse-led education on hospitalisation, readmission, quality of life and cost in adults with heart failure. A systematic review. Patient Educ Couns 101(3):363–374. https://doi.org/10.1016/j.pec.2017.10.002
Cui X, Zhou X, Ma LL, Sun TW, Bishop L, Gardiner FW, Wang L (2019) A nurse-led structured education program improves self-management skills and reduces hospital readmissions in patients with chronic heart failure: a randomized and controlled trial in China. Rural Remote Health 19(2):5270. https://doi.org/10.22605/RRH5270
Peter D, Robinson P, Jordan M, Lawrence S, Casey K, Salas-Lopez D (2015) Reducing readmissions using teach-back: enhancing patient and family education. J Nurs Adm 45(1):35–42. https://doi.org/10.1097/NNA.0000000000000155
Willson DF, Horn SD, Hendley JO, Smout R, Gassaway J (2001) Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness. Pediatrics 108(4):851–855. https://doi.org/10.1542/peds.108.4.851
Garcia-Maurino C, Moore-Clingenpeel M, Wallihan R, Koranyi K, Rajah B, Shirk T, Vegh M, Ramilo O, Mejias A (2018) Discharge criteria for bronchiolitis: an unmet need. Pediatr Infect Dis J 37(6):514–519. https://doi.org/10.1097/INF.0000000000001836
Cunningham S, Rodriguez A, Adams T, Boyd KA, Butcher I, Enderby B, MacLean M, McCormick J, Paton JY, Wee F, Thomas H, Riding K, Turner SW, Williams C, McIntosh E, Lewis SC, Bronchiolitis of Infancy Discharge Study g (2015) Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet 386(9998):1041–1048. https://doi.org/10.1016/S0140-6736(15)00163-4
Berg AS, Inchley CS, Fjaerli HO, Leegaard TM, Nakstad B (2017) Assessing severity in pediatric pneumonia: predictors of the need for major medical interventions. Pediatr Emerg Care. https://doi.org/10.1097/PEC.0000000000001179
Larsen GY, Donaldson AE, Parker HB, Grant MJ (2007) Preventable harm occurring to critically ill children. Pediatr Crit Care Med 8(4):331–336. https://doi.org/10.1097/01.PCC.0000263042.73539.99
Eulmesekian PG, Alvarez JP, Ceriani Cernadas JM, Perez A, Berberis S, Kondratiuk Y (2020) The occurrence of adverse events is associated with increased morbidity and mortality in children admitted to a single pediatric intensive care unit. Eur J Pediatr 179(3):473–482. https://doi.org/10.1007/s00431-019-03528-z
Stockwell DC, Landrigan CP, Toomey SL, Loren SS, Jang J, Quinn JA, Ashrafzadeh S, Wang MJ, Wu M, Sharek PJ, Classen DC, Srivastava R, Parry G, Schuster MA, Group GS (2018) Adverse events in hospitalized pediatric patients. Pediatrics 142(2):e20173360. https://doi.org/10.1542/peds.2017-3360
Hamrin J, Bennet R, Berner J, Rotzen-Ostlund M, Eriksson M (2021) Rates and risk factors of severe respiratory syncytial virus infection in 2008-2016 compared with 1986-1998. Acta Paediatr 110(3):963–969. https://doi.org/10.1111/apa.15575
Welliver RC (2003) Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr 143(5 Suppl):S112–S117
Freire G, Kuppermann N, Zemek R, Plint AC, Babl FE, Dalziel SR, Freedman SB, Atenafu EG, Stephens D, Steele DW, Fernandes RM, Florin TA, Kharbanda A, Lyttle MD, Johnson DW, Schnadower D, Macias CG, Benito J, Schuh S, Pediatric Emergency Research N (2018) Predicting escalated care in infants with bronchiolitis. Pediatrics 142(3):e20174253. https://doi.org/10.1542/peds.2017-4253
Ochoa Sangrador C, Gonzalez de Dios J, Research Group of the a BP (2012) Management of acute bronchiolitis in emergency wards in Spain: variability and appropriateness analysis (aBREVIADo Project). Eur J Pediatr 171(7):1109–1119. https://doi.org/10.1007/s00431-012-1683-y
Bouzas ML, Oliveira JR, Fukutani KF, Borges IC, Barral A, Van der Gucht W, Wollants E, Van Ranst M, de Oliveira CI, Van Weyenbergh J, Nascimento-Carvalho CM, Acute Respiratory Infection WSGPI II (2016) Respiratory syncytial virus a and b display different temporal patterns in a 4-year prospective cross-sectional study among children with acute respiratory infection in a tropical city. Medicine (Baltimore) 95(41):e5142. https://doi.org/10.1097/MD.0000000000005142
Broberg EK, Waris M, Johansen K, Snacken R, Penttinen P, European Influenza Surveillance N (2018) Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Euro Surveill 23(5). https://doi.org/10.2807/1560-7917.ES.2018.23.5.17-00284
Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, Rodriguez-Tenreiro C, Sly P, Ramilo O, Mejias A, Baraldi E, Papadopoulos NG, Nair H, Nunes MC, Kragten-Tabatabaie L, Heikkinen T, Greenough A, Stein RT, Manzoni P, Bont L, Martinon-Torres F (2018) Respiratory syncytial virus seasonality: a global overview. J Infect Dis 217(9):1356–1364. https://doi.org/10.1093/infdis/jiy056
Yu J, Liu C, Xiao Y, Xiang Z, Zhou H, Chen L, Shen K, Xie Z, Ren L, Wang J (2019) Respiratory syncytial virus seasonality, Beijing, China, 2007-2015. Emerg Infect Dis 25(6):1127–1135. https://doi.org/10.3201/eid2506.180532
Cangiano G, Nenna R, Frassanito A, Evangelisti M, Nicolai A, Scagnolari C, Pierangeli A, Antonelli G, Papoff P, Petrarca L, Capocaccia P, Moretti C, Midulla F (2016) Bronchiolitis: analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol 51(12):1330–1335. https://doi.org/10.1002/ppul.23476
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital). The study was fully financed by the South-East Norway Regional Health Authority.
Author information
Authors and Affiliations
Contributions
C.I., L.H., B.N., and H.F. conceived and planned the study. C.I., L.H., B.N., H.F., and C.N. planned and carried out the implementation of admission guidelines. C.I. and L.H. performed the computations. C.I., L.H., B.N., H.F., and C.N. contributed to the interpretation of the results. L.H. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the data protection officer at the hospital without need for informed consent or approval from the regional ethics committee.
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Nicole Ritz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to additional affiliation of the first author.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Havdal, L.B., Nakstad, B., Fjærli, H.O. et al. Viral lower respiratory tract infections—strict admission guidelines for young children can safely reduce admissions. Eur J Pediatr 180, 2473–2483 (2021). https://doi.org/10.1007/s00431-021-04057-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04057-4