Abstract
Despite the recent advances involving molecular studies, the neonatal cholestasis (NC) diagnosis still relays on the expertise of medical teams. Our aim was to develop models of etiological diagnosis and unfavourable prognosis which may support a rationale diagnostic approach. We retrospectively analysed 154 patients born between January 1985 and October 2019. The cohort was divided into two main groups: (A) transient cholestasis and (B) other diagnosis (with subgroups) and also in two groups of outcomes: (I) unfavourable and (II) favourable. Multivariate logistic regression analysis identified the lower gestational age as the only variable independently associated with an increased risk of transient cholestasis and signs and/or symptoms of sepsis with infectious or metabolic diseases. Gamma-glutamyl transferase serum levels > 300 IU/L had a positive predictive value for both diagnosis of biliary atresia and for alpha-1-antitrypsin deficiency (A1ATD) and for unfavourable prognosis. A model of diagnosis for A1ATD (n = 34) showed an area under the ROC curve = 0.843 [confidence interval (CI): 0.773–0.912].
Conclusion: This study identified some predictors of diagnosis and prognosis which helped to build a diagnostic decision algorithm. The unusually large subgroup of patients with A1ATD in this cohort emphasizes its predictive diagnostic model.
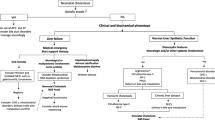
What Is Known • The etiological diagnosis of neonatal cholestasis (NC) requires a step-by-step guided approach, and diagnostic models have been developed only for biliary atresia. • Current algorithms neither address the epidemiology changes nor the application of the new molecular diagnostic tools. What Is New • This study provides diagnostic predictive models for patients with A1ATD, metabolic/infectious diseases, and transient cholestasis, and two models of unfavourable prognosis for NC. • A diagnostic decision algorithm is proposed based on this study, authors expertise and the literature. |




Similar content being viewed by others
Data Availability
The data analysed in this study is subject to the following licenses/restrictions: The data are recorded in the patients’ clinical files and in the hospital databases. In order to have access, it is necessary to ask for authorization from the Ethics Committee and the Board of Directors of the hospital. This authorization was requested and obtained to carry out this study. Requests to access these datasets should be directed to Ethics Committee, secretariado.etica@chporto.min- saude.pt, and Departement of Education, Training and Research (DEFI), secretariado.cg.defi@chporto.min-saude.pt.
References
Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, McLin V, Molleston JP, Neimark E, Ng VL, Karpen SJ (2017) Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 64(1):154–168
Hoerning A, Raub S, Dechene A et al (2014) Diversity of disorders causing neonatal cholestasis - the experience of a tertiary pediatric center in Germany. Front Pediatr 2:65
Santos Silva E, Almeida A, Frutuoso S, Martins E, Valente MJ, Santos-Silva A, Lopes AI (2020) Neonatal cholestasis over time: changes in epidemiology and outcome in a cohort of 154 patients from a portuguese tertiary center. Front Pediatr 8:351
Jacquemin E, Lykavieris P, Chaoui N, Hadchouel M, Bernard O (1998) Transient neonatal cholestasis: origin and outcome. J Pediatr 133(4):563–567
van Dijk EL, Jaszczyszyn Y, Naquin D, Thermes C (2018) The third revolution in sequencing technology. Trends Genet 34(9):666–681
Togawa T, Sugiura T, Ito K et al (2016) Molecular genetic dissection and neonatal/infantile intrahepatic cholestasis using targeted next-generation sequencing. J Pediatr 171:171–7.e1-4. https://doi.org/10.1016/j.jpeds.2016.01.006
Nicastro E, Di Giorgio A, Marchetti D et al (2019) Diagnostic yield of an algorithm for neonatal and infantile cholestasis integrating next generation sequencing. J Pediatr 211:54–62.e4
Dong R, Jiang J, Zhang S, Shen Z, Chen G, Huang Y, Zheng Y, Zheng S (2018) Development and validation of novel diagnostic models for biliary atresia in a large cohort of chinese patients. EBioMedicine 34(34):223–230
Shneider BL, Moore J, Kerkar N, Magee JC, Ye W, Karpen SJ, Kamath BM, Molleston JP, Bezerra JA, Murray KF, Loomes KM, Whitington PF, Rosenthal P, Squires RH, Guthery SL, Arnon R, Schwarz KB, Turmelle YP, Sherker AH, Sokol RJ, for the Childhood Liver Disease Research Network (2017) Initial assessment of the infant with neonatal cholestasis —Is this biliary atresia? PLoS One 12(5):e0176275
Lee SM, Cheon J-E, Choi YH, Kim WS, Cho HH, Kim IO, You SK (2015) Ultrasonographic diagnosis of biliary atresia based on a decision-making tree model. Korean J Radiol 16(6):1364–1372
Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, Heyman MB, North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (2004) Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 39(2):115–128
Champion V, Carbajal R, Lozar J, Girard I, Mitanchez D (2012) Risk factors for developing transient neonatal cholestasis. J Pediatr Gastroenterol Nutr 55(5):592–598
Satrom K, Gourley G (2016) Cholestasis in Preterm Infants. Clin Perinatol 43(2):355–373
Squires RH, Shneider B, Bucuvalas J et al (2006) Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr 148(5):652–658
Moreira-Silva H, Maio I, Bandeira A, Gomes-Martins E, Santos-Silva E (2019) Metabolic liver diseases presenting with neonatal cholestasis: at the crossroad between old and new paradigms. Eur J Pediatr 178(4):515–523
Feldman AG, Sokol RJ (2019) Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics. Nat Rev Gastroenterol Hepatol 16(6):346–360
Vilarinho L, Rocha H, Sousa C et al (2010) Four years of expanded newborn screening in Portugal with tandem mass spectrometry. J Inherit Metab Dis 33(Suppl 3):S133–S138
Gottesman LE, Del Vecchio MT, Aronoff SC (2015) Etiologies of conjugated hyperbilirubinemia in infancy: a systematic review of 1692 subjects. BMC Pediatr 15:192
Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, Doo E, Sokol RJ (2018) Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology 68(3):1163–1173
Tufano M, Nicastro E, Giliberti P, Vegnente A, Raimondi F, Iorio R (2009) Cholestasis in neonatal intensive care unit: incidence, aetiology and management. Acta Paediatr 98(11):1756–1761
Hsiao CH, Chang MH, Chen HL, Lee HC, Wu TC, Lin CC, Yang YJ, Chen AC, Tiao MM, Lau BH, Chu CH, Lai MW, Taiwan Infant Stool Color Card Study Group (2008) Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology 47(4):1233–1240
Lu FT, Wu JF, Hsu HY, Ni YH, Chang MH, Chao CI, Chen HL (2014) Gamma-glutamyl transpeptidase level as a screening marker among diverse etiologies of infantile intrahepatic cholestasis. J Pediatr Gastroenterol Nutr 59(6):695–701
Copple BL, Jaeschke H, Klaassen CD (2010) Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis 30(2):195–204
Kosters A, Karpen SJ (2010) The role of inflammation in cholestasis: clinical and basic aspects. Semin Liver Dis 30(2):186–194
Masubuchi N, Sugihara M, Sugita T, Amano K, Nakano M, Matsuura T (2016) Oxidative stress markers, secondary bile acids and sulfated bile acids classify the clinical liver injury type: Promising diagnostic biomarkers for cholestasis. Chem Biol Interact 255:83–91
Dong R, Shen Z, Zheng C, Chen G, Zheng S (2016) Serum microRNA microarray analysis identifies miR-4429 and miR-4689 are potential diagnostic biomarkers for biliary atresia. Sci Rep 6:21084
Zahm AM, Hand NJ, Boateng LA et al (2012) Circulating MicroRNA is a biomarker of biliary atresia. J Pediatr Gastroenterol Nutr 55(4):366–369
Acknowledgements
Many thanks to Dr Margarida Medina (emeritus paediatrician of Hospital Geral de Santo António, and Hospital de Crianças Maria Pia, Porto, Portugal) for providing and caring for many study patients.
Funding
This work was supported by a PhD scholarship from the Department of Education, Training, and Research of the Centro Hospitalar Universitário do Porto, and by Applied Molecular Biosciences Unit (UCIBIO), which is financed by national funds from FCT/MCTES (UID/MULTI/04378/2019).
Author information
Authors and Affiliations
Contributions
ESS diagnosed and followed patients, designed the study, collected and analysed data, and elaborated the draft of the manuscript.
HMS collected and analysed data, contributed to the design of the study, and critically reviewed the manuscript.
CC analysed data and critically reviewed the manuscript.
CCD built and helped to interpret the predictive models of diagnosis and prognosis, revised all statistical analysis, and critically reviewed the manuscript.
ASS and AIL contributed to the conception of the study and the interpretation of data and critically reviewed the manuscript.
All authors approved the final version of the manuscript, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was in accordance with the ethical standards of the participating healthcare institution committee (Studies N/REF.ª 2016. 081 (069-DEFI/066-CES) and N/REF.ª 2016. 084 (072-DEFI/069-CES)), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individuals included in the study.
Consent for publication
Informed consent was obtained from all individuals included in the study.
Code availability
Software Statistical Package for the Social Sciences v. 24.0.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Online Resource 1
Clinical and biochemical variables tested in the predictive models (PDF 35 kb)
Online Resource 2
Detailed description of underlying entities. Legend: CDG, congenital disorders of glycosylation; PFIC, progressive familial intra-hepatic cholestasis; A1ATD, alpha-1-antitrypsin deficiency; UTI, urinary tract infection. (PDF 52 kb)
Online Resource 3
Participants flow diagram (PDF 240 kb)
Online Resource 4
Participants characteristics (I): transient cholestasis versus all other diagnosis. (PDF 76 kb)
Online Resource 5
Overall survival: transient cholestasis versus other diagnosis. (PDF 63 kb)
Online Resource 6
Participants characteristics (II): comparison between some subgroups of “other diagnosis”. (PDF 95 kb)
Online Resource 7
Participants characteristics (III): unfavourable versus favourable outcome. (PDF 73 kb)
Rights and permissions
About this article
Cite this article
Santos Silva, E., Moreira Silva, H., Catarino, C. et al. Neonatal cholestasis: development of a diagnostic decision algorithm from multivariate predictive models. Eur J Pediatr 180, 1477–1486 (2021). https://doi.org/10.1007/s00431-020-03886-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03886-z