Abstract
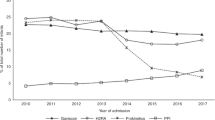
Non-specific symptoms such as irritability, vomiting, and back arching during the infant period are often attributed to gastroesophageal reflux. While numerous studies have shown no significant benefit to the use of acid suppressant medications in this population, these medications are frequently prescribed in response to these symptoms. Our goals were to understand how often children were being prescribed this medication. To do this, data was extracted from a national database for reimbursement of prescribed medications through the General Medical Services scheme (GMS). Infants aged less than 1 year and eligible for reimbursement under GMS were included for analysis. A total of 450 infants per 10,000 eligible population received an anti-reflux preparation from the following drug classes (H2 antagonists, proton pump inhibitors, or alginate preparations) in 2018. This is compared with that in 2009 where only 137 per 10,000 eligible infants received these medications. This increase was predominantly attributable to an increase in ranitidine prescriptions.
Conclusion: Despite a change in clinical guidelines, anti-reflux preparations are increasingly being prescribed to infants aged less than 1 year. The reasons behind the increase in prescriptions containing these medications cannot be ascertained from this data. This may suggest a proportion of these prescriptions may be unwarranted in this population.
What is Known: • The prescription of PPIs in infants has increased in a number of countries. • Use of anti-reflux medications has a very poor evidence base in infancy. What is New: • This data focuses only on an infant age group in a “well” cohort. • Ranitidine may contribute to increased acid-suppressant use in infancy. |

Similar content being viewed by others
Abbreviations
- PPI:
-
Proton pump inhibitor
- H2RAs:
-
Histamine type 2 receptor antagonists
- GORD:
-
Gastroesophageal reflux disease
- CMPI:
-
Cow’s milk protein intolerance
- CSO:
-
Central Statistics Office
- GMS:
-
General Medical Services Scheme
- HSE:
-
Health Service Executive
- PCRS:
-
Primary care reimbursement service
- ESPHAGN:
-
European Society for Gastroenterology, Hepatology and Nutrition
- NASPHAGN:
-
North American Society for Gastroenterology, Hepatology and Nutrition
- NICE:
-
National Institute of Clinical Excellence
References
Singendonk M, Goudswaard E, Langendam M, van Wijk M, van Etten-Jamaludin F, Benninga M, Tabbers M (2019) Prevalence of gastroesophageal reflux disease symptoms in infants and children: a systematic review. J Pediatr Gastroenterol Nutr 68:811–817. https://doi.org/10.1097/MPG.0000000000002280
Nelson SP, Chen EH, Syniar GM, Christoffel KK (1997) Prevalence of Symptoms of gastroesophageal reflux during infancy: a pediatric practice based survey. Arch Pediatr Adolesc Med 151:569–572
Rosen R, Vandenplas Y, Singendonk M et al (2018) Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN). JPGN 66:516–554. https://doi.org/10.1016/j.physbeh.2017.03.040
Shin MS, Shim JO, Moon JS, Kim HS, Ko JS, Choi JH, Seo JK (2012) Impedance-pH monitoring and conventional pH monitoring are complementary methods to detect association between gastroesophageal reflux and apnea-related symptoms in preterm infants and neonates. J Matern Fetal Neonatal Med 25:2406–2410. https://doi.org/10.3109/14767058.2012.697944
Condino AA, Sondheimer J, Pan Z, Gralla J, Perry D, O’Connor JA (2006) Evaluation of infantile acid and nonacid gastroesophageal reflux using combined pH monitoring and impedance measurement. J Pediatr Gastroenterol Nutr 42:16–21. https://doi.org/10.1097/01.mpg.0000188008.66752.72
Gieruszczak-Białek D, Konarska Z, Skórka A et al (2015) No effect of proton pump inhibitors on crying and irritability in infants: systematic review of randomized controlled trials. J Pediatr 166:767–770.e3. https://doi.org/10.1016/j.jpeds.2014.11.030
Tighe M, Afzal NA, Bevan A, Hayen A, Munro A, Beattie RM, Cochrane Upper GI and Pancreatic Diseases Group (2014) Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008550.pub2www.cochranelibrary.com
National Institute for health and Care Excellence (NICE) (2015) Gastrooesophageal reflux disease in children and young people: Diagnosis and management
Malchodi L, Wagner K, Susi A, Gorman G, Hisle-Gorman E (2019) Early acid suppression therapy exposure and fracture in young children. Pediatrics 144:e20182625. https://doi.org/10.1542/peds.2018-2625
Canani RB, Cirillo P, Roggero P, Malamisura B (2006) Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics 117:e817–e820. https://doi.org/10.1542/peds.2005-1655
Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM (2018) Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr 172:e180315. https://doi.org/10.1001/jamapediatrics.2018.0315
De Bruyne P, Christiaens T, Vander SR, Van Winckel M (2014) Changes in prescription patterns of acid-suppressant medications by belgian pediatricians: Analysis of the national database, [1997-2009]. J Pediatr Gastroenterol Nutr 58:220–225. https://doi.org/10.1097/MPG.0b013e3182a3b04e
Blank ML, Parkin L (2017) National study of off-label proton pump inhibitor use among New Zealand infants in the first year of life (2005-2012). J Pediatr Gastroenterol Nutr 65:179–184. https://doi.org/10.1097/MPG.0000000000001596
Barron JJ, Tan H, Spalding J, Bakst AW, Singer J (2007) Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr 45:421–427. https://doi.org/10.1097/MPG.0b013e31812e0149
Illueca M, Alemayehu B, Shoetan N, Yang H (2014) Proton pump inhibitor prescribing patterns in newborns and infants. J Pediatr Pharmacol Ther 19:283–287. https://doi.org/10.5863/1551-6776-19.4.283
Aznar-Lou I, Reilev M, Lødrup AB, Rubio-Valera M, Haastrup PF, Pottegård A (2019) Use of proton pump inhibitors among Danish children: a 16-year register-based nationwide study. Basic Clin Pharmacol Toxicol 124:704–710. https://doi.org/10.1111/bcpt.13191
Jung AD (2001) Gastroesophageal reflux in infants and children. Am Fam Physician 64:1853–1860. https://doi.org/10.3109/00365527109180710
Mc Namara A, Normand C, Whelan B (2013) Patterns and determinants of health care utilisation in Ireland. The longitudinal study on aging. https://doi.org/10.38018/TildaRe.2013-00
Hassall E (2012) Over-prescription of acid-suppressing medications in infants: how it came about, why it’s wrong, and what to do about it. J Pediatr 160:193–198. https://doi.org/10.1016/j.jpeds.2011.08.067
Acknowledgments
The authors thank David Stratton and Ivan McConkey, the Health Service Executive, and the Primary Care Reimbursement Service.
Author information
Authors and Affiliations
Contributions
DOR: Generated hypothesis, designed study, drafted manuscript and approved final manuscript RC: Designed study, statistical analysis, drafted manuscript and approved final manuscript LOC: Refined study design, statistical analysis, refined manuscript and approved final manuscript PF: Generated hypothesis, refined manuscript and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any experiments with human participants or animals performed by any of the authors. It was performed retrospectively and using data which was already anonymized.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Reilly, D., Conway, R., O’Connor, L. et al. Use of anti-reflux medications in infants under 1 year of age: a retrospective drug utilization study using national prescription reimbursement data. Eur J Pediatr 179, 1963–1967 (2020). https://doi.org/10.1007/s00431-020-03837-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03837-8