Abstract
Osteogenesis imperfecta (OI) is a rare genetic disorder of type I collagen. Type I is the most common, which is called a non-deforming type of OI, as in this condition, there are no major bone deformities. This type is characterised by blue sclera and vertebral fractures, leading to mild scoliosis. The body height of these patients is regarded as normal, or only slightly reduced, but there are no data proving this in the literature. The aim of this study is the preparation of the developmental charts of children with OI type I. The anthropometric data of 117 patients with osteogenesis imperfecta were used in this study (61 boys and 56 girls). All measurements were pooled together into one database (823 measurements in total). To overcome the problem of the limited number of data being available in certain age classes and gender groups, the method called reverse transformation was used. The body height of the youngest children, aged 2 and 3 years, is less than that of their healthy peers. Children between 4 and 7 years old catch up slightly, but at later ages, development slows down, and in adults, the median body height shows an SDS of −2.7.
Conclusion: These results show that children with type I OI are smaller from the beginning than their healthy counterparts, their development slows down from 8 years old, and, ultimately, their body height is impaired.
What is Known: |
• The body height of patients with osteogenesis imperfecta type I is regarded as normal, or only slightly reduced, but in the known literature, there is no measurement data supporting this opinion. |
What is New: |
• Children with type I osteogenesis imperfecta are smaller from the beginning than their healthy counterparts, their development slows down from 8 years old and, ultimately, their final body height is impaired. • The developmental charts for the body height, body weight and BMI of children with type I osteogenesis imperfecta are shown. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteogenesis imperfecta (OI) is a rare genetic disorder of type I collagen. Its frequency is estimated as 1 in 20,000. There are several forms of this disease. Sillence [10] proposed a classification into four types, based on radiographic, clinical and genetic data; in some studies, even more are described [3, 7]. The most common is type I, which is called a non-deforming type of OI [7], as in this condition, there are no major bone deformities. This type is characterised by blue sclera and vertebral fractures, leading to mild scoliosis. Functional level and intellectual development, as well as life expectancy, are normal. As this type is the mildest of all OI types, the body height of these patients is acknowledged as normal or only slightly reduced [3]. But in the known literature, there is no measurement data supporting this opinion.
The assessment of body height and growth is a very important measure of the state of health of children, and paediatric charts are universally used [8]. Abnormal body height and growth (height velocity) could be symptoms of an underlying disease and need further diagnostics and attention [4]. This information on body height and growth should be easily accessible to paediatricians in monitoring their patients’ condition. Children with many genetic disorders experience affected growth and body height, so using the charts of healthy children is unreliable and could lead to the overlooking of some secondary problems also affecting body height and growth. In the literature, there are studies which prepared the growth charts of children with specific disorders to aid the detection of additional problems influencing growth patterns [2, 5, 11].
The aim of the present study is the preparation of the developmental charts of children with osteogenesis imperfecta, type I.
Materials and methods
The anthropometric data of 117 patients with osteogenesis imperfecta type I according to the Sillence classification [10, 11] were used in this study (61 boys and 56 girls). The classification of OI patients into type I was retrospectively checked through the analysis of medical documentation and updated classification criteria. These children were being treated for their primary disease in The Children’s Memorial Health Institute and were being regularly measured. Children with comorbidities which could influence their body development were excluded from the database. Also, the measurements of patients who were undergoing surgical interventions for bone trauma (due to accidental fractures) were excluded from the database. The patients in our group neither required rodding nor had developed deformities which needed surgical correction. None of the patients had scoliosis or vertebral compression fractures. None of the patients had received bisphosphonates. All measurements were pooled together into one database (823 measurements in total). The number of measurements per patient varied from 1 to 35, with a median of 5 measurements per patient. The youngest patient who was measured was 4 months old; the oldest 22 years old. The data for patients older than 18 were treated as the time point of 18 years old. Because of the very limited number of data related to children less than 2 years of age, the charts were prepared for ages from 2 to 18 years. For children older than 1 year, body height was measured using an anthropometer. They stood with an upright posture looking straight ahead, with both legs and feet together, knees and legs straight, shoulders relaxed and arms by their sides. The accuracy of the measurement was within approximately 0.1 mm.
To overcome the problem of the limited number of data being available for certain age and gender groups, the method called reverse transformation, previously used to prepare the development charts of children with achondroplasia, was applied [2]. This method is described in Appendix 1. In the first step, the individual data of each patient were converted into a number. This number represented the difference between the raw score and the population mean in terms of the population’s standard deviation. The data (body height, body weight and BMI) of the healthy Polish population of children and adolescents (body height, body weight and BMI) were used as a reference population database [9].
A statistical analysis was performed using Statistica, v.10.0 (StatSoft), and regression curves were prepared using Matlab software. The Student t test was used for comparisons and the Spearman rank-correlation coefficient for checking the dependence between age and the analysed variables.
Results
Body height
As there was no statistically significant difference between boys and girls in normalised body height (Student’s t test p = 0.777), the data were pooled together. The correlation coefficient showed the dependence of normalised body height on age (R = −0.293, p < 0.005). Therefore, from the pooled database, the median, the upper and lower quartile and the 10th and 90th percentiles were calculated for the normalised body height (Tab. I in the Supplementary material). From these data, reverse transformation facilitated the calculation of the median, the upper and lower quartiles and the 10th and 90th percentiles of age groups for boys and girls separately (Table 1 and Table 2).
The regression equation describing the regression curves for the median, the lower and upper quartiles and the 10th and 90th percentiles for body height was
The constants a1, a2 and a3 for the regression equations for boys and girls are presented separately in Tables II and III in the Supplementary material.
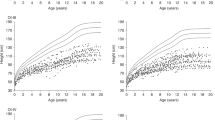
Figure 1 shows the developmental charts of body height for children with type I osteogenesis imperfecta—(a) boys and (b) girls.
Developmental charts for the body height of children with type I osteogenesis imperfecta (black): a boys and b girls, against reference data on the healthy population (grey) [8]
Body weight
As there was no statistically significant difference between boys and girls in normalised body weight (Student t test t = −1.251, p = 0.211), the data were pooled together. The correlation coefficient showed the weak dependence of normalised body weight on age (R = −0.138, p < 0.005). Therefore, from the pooled database median, the upper and lower quartiles and 10th and 90th percentiles were calculated for the normalised body weight (Tab. IV in the Supplementary material). From these data, the reverse transformation facilitated the calculation of the median, the upper and lower quartiles and the 10th and 90th percentiles of the age groups for boys and girls separately (Table 3 and Table 4).
The regression equation describing the regression curves for the median, the lower and upper quartiles and 10th and 90th percentiles for body weight was
The constants a1, a2 and a3 for the regression equations for boys and girls are presented separately in Tables V and VI in the Supplementary material.
Figure 2 shows the developmental charts of body weight for children with type I osteogenesis imperfecta—(a) boys and (b) girls.
Developmental charts for the body mass of children with type I osteogenesis imperfecta (black): a boys and b girls, against reference data on the healthy population (grey) [8]
BMI
As there was a statistically significant difference between boys and girls in normalised BMI (Student’s t test = −2.839, p = 0.005), the data could not be pooled together. The correlation coefficient in both gender groups showed no dependence of normalised BMI on age (R = 0.031, p > 0.05 for boys and R = 0.023, p > 0.005 for girls). As there was no dependence on the age median or the upper and lower quartiles, the 10th and 90th percentiles were calculated for normalised BMI separately for boys and girls (Table VII Supplementary material). From these data, reverse transformation facilitated the calculation of the median, the upper and lower quartiles and the 10th and 90th percentiles of the age groups for boys and girls separately (Tables VIII and IX Supplementary material).
The regression equation describing the regression curves for the median, the lower and upper quartiles and the 10th and 90th percentile BMI was
The constants a1, a2 and a3 for the regression equations for boys and girls are presented separately in Tables X and XI in the Supplementary material.
Figure 3 shows the developmental BMI charts for children with type I osteogenesis imperfecta—(a) boys and (b) girls.
Developmental BMI charts for children with type I osteogenesis imperfecta (black): a boys and b girls, against reference data on the healthy population (grey) [8]
Discussion
Syndrome-specific developmental charts have proved to be helpful in medical practice [2, 8, 12]. Children with various syndromes could suffer from other comorbidities, which also negatively influence their development. Without the proper reference database, it is difficult to decide whether the impaired growth is being caused by primary disease or also by secondary diseases. In the case of rare diseases, it is difficult to compile enough measurements during the developmental process to be able to create proper developmental charts.
In this study, we used the so-called reversed transformation method developed for the construction of developmental charts for another rare disease—achondroplasia [2]. This method, together with regression equations, enabled the construction of developmental charts for boys and girls of 2 to 18 years with type I osteogenesis imperfecta. For rare diseases, it is difficult to collect enough data broken down by gender and age groups to construct developmental charts. Therefore, some alternatives must be found. In some cases, the data were gathered from various sources and literature [12]. Our method is an alternative which can be used when there is an insufficient number of subjects. This method has a drawback: as the curves are calculated using regression equations, the pubertal growth spurt is smoothed and does not stand out; this is the limitation of such a measure.
This type of OI is the mildest one—patients do not suffer from bone deformations, and their body height is regarded as normal, or only slightly reduced. Our results show that the body height of the youngest children, aged 2 or 3 years, is less than their healthy peers (the median is an SDS of −1.2 in the case of 2-year-olds, and an SDS of −0.9 in the case of 3-year-olds). Older children, between 4 and 7 years old, catch up slightly, and their median body height is around an SDS of −0.5, but at later ages, the development slows down, and in adults, the median body height exhibits an SDS of −2.7. These results are consistent with the results of the study of Aglan et al. [1]. Their study included 124 OI patients, but only 16 with OI type I, the age range being from 0.9 to 10.75 years. The mean height of these patients was an SDS of −0.426. Even the tallest OI type I patients (the 90th percentile) were smaller than their average healthy peers (an SDS of −0.5). The longitudinal study of Germain-Lee [6] on 36 patients with OI type I patients showed that their final body height was reduced in comparison with their healthy peers. These results show that children with type I OI are smaller from the beginning than their healthy counterparts, their development slows down from 8 years old and ultimately, their body height is impaired.
A similar trend can be observed in the case of body weight, inasmuch as the ratio between body height and body weight in type I OI patients is similar to that in healthy subjects. This fact is reflected in the body mass index (BMI) which is similar in OI patients to the BMI of healthy children and adolescents.
The patients in this study were classified into type I OI according to the Sillence classification [10, 11], which is based on the phenotype. As this was a retrospective study, in the case of the majority of the patients, there were no data on their genotype.
Abbreviations
- BMI:
-
Body mass index
- OI:
-
Osteogenesis imperfecta
- SDS:
-
Standard deviation score
References
Aglan MS, Zaki ME, Hosny L, El-Houssini R, Oteify G, Temtamy SA (2012) Anthropometric measurements in Egyptian patients with osteogenesis imperfecta. Am J Med Genet Part A 158A:2714–2718
Arasimowicz E, Syczewska M (2008) A method for prediction of growth in children with achondroplasia. Endokrynol Diabetol Choroby Przemiany Materii Wieku Rozw 14:237–241
Bishop N (2010) Characterizing and treating osteogenesis imperfecta. Early Hum Dev 86:743–746
Bridges N (2013) Growth and puberty. Medicine 41:600–603
Gawlik A, Gawlik T, Augustyn M, Woska W, Malecka-Tendera E (2006) Validation of growth charts for girls with Turner syndrome. Int J Clin Pract 60:150–155
Germain-Lee EL, Brennen FS, Stern D, Kantipuly A, Melvin P, Terkowitz MS, Shapiro JR (2016) Cross-sectional and longitudinal growth patterns in osteogenesis imperfecta: implications for clinical care. Pediatr Res 79:489–495
Michell C, Patel V, Amirfeyz R, Gargan M (2007) Osteogenesis imperfecta. Curr Orthop 21:236–241
de Onis M, Wijnhoven TMA, Onyango AW (2004) Worldwide practices in child-growth monitoring. J Pediatr 144:461–465
Palczewska I, Niedzwiecka Z (2001) Wskazniki rozwoju somatycznego dzieci i młodziezy warszawskiej. Medycyna Wieku Rozwojowego V (no 2), Suppl.1 (in Polish)
Sillence DA, Senn A, Danks DM (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Gent 16:91–116
Van Dijk FS, Sillence DO (2014) Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet Part A 164A:1470–1481
Verbeek S, Eilers PH, Lawrence K, Hennekam RCM, Versteegh FGA (2011) Growth charts for children with Ellis-van Creveld syndrome. Eur J Pediatr 170:207–211
Authors’ contributions
Krzysztof Graff—conception and design of the study, data acquisition (patients’ measurements), management of database, preparation of the manuscript, finding relevant references and final approval of the manuscript.
Malgorzata Syczewska—conception and design of the study, management of the database, analysis of the data, preparation of tables and charts, preparation of the manuscript, finding relevant references and final approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was an opportunistic sample study in which anonymised data were extracted from a clinical database. All patients were being treated for OI as the primary disease, and body measurements were part of the clinical procedure. The database covered the years 1974–2013.
Funding
None.
Ethical approval
All the procedures performed in the studies involving patients were in accordance with the ethical standards of the institution on clinical practice and with the 1964 Helsinki Declaration, as amended. The parents or legal guardians of patients signed informed-consent forms (when such a requirement was introduced in Poland) in which they agreed to the treatment and all the diagnostic procedures required.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Mario Bianchetti
Revisions received: 18 October 2016; 20 December 2016
Electronic supplementary material
Appendix 1
Appendix 1
The individual data on each patient was standardised according to the following equation.
where
- RSDS :
-
the standardised results of the patient (body height, body weight or BMI)
- Ri :
-
the individual measurement of the patient (body height, body weight or BMI, respectively)
- RN :
-
the mean value of the age- and gender-matched reference database for a given variable (body height, body weight or BMI, respectively)
- SDN :
-
the standard deviation for the age- and gender-matched reference database for a given variable (body height, body weight or BMI, respectively)
To create the developmental charts, data from Tables I, VI and XI (standardised values) were transformed according to the following equation:
where
- -Xi :
-
variable (body height, body weight or BMI) for the developmental chart
- -Xjk :
-
the mean for the j-age and k-gender of the variable from the reference database
- -Ws :
-
the standardised percentile for the given age of the variable (from Tables I, VI or XI, respectively)
- -Sjk :
-
the standard deviation for the j-age and k-gender of the variable from the reference database
The reference database for this study was reference data on healthy Polish children and adolescents [8].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Graff, K., Syczewska, M. Developmental charts for children with osteogenesis imperfecta, type I (body height, body weight and BMI). Eur J Pediatr 176, 311–316 (2017). https://doi.org/10.1007/s00431-016-2839-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2839-y