Abstract
Our aims were to determine whether volume-targeted ventilation (VTV) or pressure-limited ventilation (PLV) reduced the time to successful extubation and if any difference was explained by a lower work of breathing (WOB), better respiratory muscle strength or less thoracoabdominal asynchrony (TAA) and associated with fewer hypocarbic episodes. Infants born at ≥34 weeks of gestational age were randomised to VTV or PLV. The WOB was assessed by the transdiaphragmatic pressure time product, respiratory muscle strength by the maximum inflation (Pimax) and expiratory (Pemax) pressures and TAA assessed using uncalibrated respiratory inductance plethysmography. Forty infants, median gestational age of 39 (range 34–42) weeks, were recruited. The time to successful extubation did not differ between the two groups (median 25, range 2.5–312 h (VTV) versus 33.5, 1.312 h (PLV)) (p = 0.461). There were no significant differences between the groups with regard to the WOB, respiratory muscle strength or the TAA results. The median number of hypocarbic episodes was 1.5 (range 0–8) in the VTV group versus 4 (range 1–13) in the PLV group (p = 0.005).
Conclusion: In infants born at or near term, VTV compared to PLV did not reduce the time to successful extubation but was associated with significantly fewer hypocarbic episodes.
What is known: |
• In prematurely born infants, volume-targeted ventilation (VTV) compared to pressure-limited ventilation (PLV) reduces bronchopulmonary dysplasia or death. |
• In addition, VTV is associated in prematurely born infants with lower incidences of pneumothorax, intraventricular haemorrhage and hypocarbic episodes. |
What is new: |
• Despite a high morbidity, few studies have investigated optimum ventilation strategies for infants born at or near term. |
• In a RCT, we have demonstrated VTV versus PLV in infants ≥34 weeks gestation was associated with significantly fewer hypocarbic episodes. |
Similar content being viewed by others
Introduction
It has been estimated that 3.6 per 1000 infants born at term require mechanical ventilation [9]. Approximately 35,000 infants per annum in the USA alone require mechanical ventilation secondary to hypoxic respiratory failure at or near term [8]. The mortality rate amongst ventilated term-born infants ranges from 9.1 to 11.7 % [1] and has been quoted to be high (9.6 to 12.2 %) even amongst infants without congenital anomalies [14, 20]. Morbidity in ventilated infants born at or near term is common [8, 18]. Yet few studies have examined or compared ventilatory modes in such infants [4]. During volume-targeted ventilation (VTV), a relatively constant volume is delivered with each ventilator inflation regardless of changes in the infant’s lung function. A meta-analysis of results of randomised controlled trials (RCTs) demonstrated that VTV compared to pressure-limited ventilation (PLV) was associated with significant reductions in death or BPD, pneumothorax, periventricular leukomalacia (PVL), grade III–IV intraventricular haemorrhage (IVH) and episodes of hypocarbia [21]. In addition, the duration of ventilation was significantly shorter in infants supported by VTV [21]. A more recently reported meta-analysis demonstrated a significant reduction in BPD [17]. None of the trials in the systematic reviews [17, 21], however, included infants born at or near term, and it is, therefore, unclear whether VTV would benefit such a population.
In the RCTs [17, 21], a wide range of volume target (VT) levels was used (4–10 ml/kg) [17, 21]. We have demonstrated in infants born at or near term, the level of VT influences, the work of breathing (WOB) with higher levels resulting in a lower WOB [6]. Increasing the level of respiratory support by increasing the VT level, however, could unfavourably impact on respiratory muscle strength. It is also not clear whether VTV or PLV would be associated with lower thoracoabdominal asynchrony (TAA) or fewer episodes of hypocarbia in infants born at or near term. Our aim, therefore, was to undertake a randomised study of PLV and VTV in infants born at or near term to determine which modality was associated with a shorter time to extubation, whether this was explained by differences in the WOB, respiratory muscle strength or TAA and associated with fewer episodes of hypocarbia.
Methods
A randomised trial was carried out at King’s College Hospital NHS Foundation Trust between May 2011 and October 2014. Infants born at 34 weeks or more of gestational age who were less than 2 weeks of age were eligible for entry into the trial if they had been ventilated for less than 24 h. Infants with congenital diaphragmatic hernia and infants who were supported by high-frequency oscillatory ventilation (HFOV) were ineligible. Infants were enrolled into the study if their parents gave informed written consent. The study was approved by King’s College Hospital Research Ethics Committee.
Patients were randomised using sequential opaque sealed envelopes and random number table generation to receive either VTV or PLV. The infants in both arms of the trial were supported by SLE 5000 ventilators (software versions 4.3; SLE Ltd., South Croydon, UK). All infants were ventilated via shouldered endotracheal tubes with minimal or non-existent leaks [11]. All infants had indwelling intra-arterial lines. The unit’s standard protocol was to use PLV during acute respiratory distress that is inflation times of 0.3–0.4 s, rates of 40–60 breaths per minute (bpm) manipulated to try to achieve synchrony and peak pressures to achieve appropriate arterial carbon dioxide (PaCO2) levels between 4.5 and 7 kPa providing the pH was above 7.25. The unit’s standard policies were for ventilated infants regardless of ventilation mode to receive intravenous morphine if they were asynchronous with ventilator inflations and post-operatively for pain relief. At randomisation, no changes were made to the ventilator settings of infants who were to receive PLV. For those randomised to VTV, the VT level was set at 5 ml/kg with the leak compensation at 20 %. The maximum peak inspiratory pressure (PIP) was set at 5 cmH2O above the PIP used during the previous ventilation mode to allow a volume delivery of 5 ml/kg. The PIP was increased by 1–2 cmH2O as necessary until the desired volume was delivered. During VTV with an SLE 5000 ventilator, the maximum set peak inflation was delivered to the infant only if the VT level was not achieved. Using the SLE ventilator, inflation was terminated once the VT level was achieved, which meant that the delivered inflation time might be shorter than the preset inflation time. If the delivered inflation time was noted to be less than 0.2 s, it was planned that the waveform would be altered to give a shallower upstroke to the inflating pressure to prolong the inflation time, but this was not required for any of the study population.
If infants developed a respiratory acidosis on VTV, the rate was increased in steps of 5 up to 60 bpm and, if that was not associated with resolution of the respiratory acidosis, then the VT level was increased in steps of 0.5 up to a maximum of 6 ml/kg. If infants developed a respiratory acidosis on PLV, the rate was increased in steps of 5 to a maximum of 60 bpm, and if necessary, the pressure was increased up to a maximum of 30 cmH2O. If those manoeuvres did not bring about the desired improvement in blood gases, the infant was transferred to HFOV. Infants were deemed to have failed the randomised mode if they required HFOV or a PIP >30 cmH2O or had a pulmonary haemorrhage (diagnosed if there was fresh blood from the endotracheal tube associated with clinical deterioration).
Infants were weaned on PLV mode by first reducing the pressure to 18 cmH2O and then the rate to a minimum of 20 bpm. On VTV mode, first the tidal volume was reduced to 5 ml/kg (if a higher level had been used) and the rate to a minimum of 20 bpm. On both modes, infants were extubated when the rate had been reduced to 20 bpm. Infants were extubated into the appropriate concentration of oxygen; non-invasive respiratory support was not used.
Measurements of the WOB, respiratory muscle strength and TAA were performed prior to extubation. The WOB was assessed over a 5-min period by measurement of the transdiaphragmatic pressure time product (PTPdi) as previously described [6]. Respiratory muscle strength was assessed by measuring the maximum inflation (Pimax) and maximum expiratory pressure (Pemax) generated during an airway occlusion during crying as previously described [6]. Thoracoabdominal synchrony was assessed using uncalibrated respiratory inductance plethysmography (Respitrace model 10.9230, Ambulatory Monitoring, New York, USA) in AC-coupled mode. Inductance coils embedded in two elastic bandages were placed around the ribcage (RC) and mid-abdomen. For each breath, the RC and abdominal wall (AB) movements were derived from the recording software. A Lissajous figure was plotted and asynchrony between RC and abdominal motion quantified. The phase angle was determined by comparing the difference between inspiratory and expiratory abdominal positions at mid-RC excursion (ABdiff) with the maximum abdominal excursion (ABmax). The phase angle ϕ was calculated as sin ϕ = ABdiff/ABmax. Oxygen saturation was monitored throughout the measurements. The clinicians caring for the infants were blinded to the results of the physiological measurements.
The nurses recorded hourly the level of respiratory support on observation charts. Arterial blood gas results were also recorded on the observation charts, and from those data, the number of episodes of hypocarbia (PaCO2 < 4.5 Kpa) experienced by each infant was determined. Arterial blood sampling was undertaken for clinical purposes. The infant’s demographics and pre-extubation levels of respiratory support were identified from the medical records and intensive care observation charts.
The primary outcome was the time from randomisation to the first successful extubation. The timing of extubation was determined by the clinical team unaware of the research team’s physiological measurement results. Successful extubation was defined as the infant remained extubated for at least 48 h. Infants were reintubated according to unit’s routine criteria: a major apnoea, frequent apnoeas with bradycardia or development of a severe respiratory acidosis (pH < 7.20).
Statistical analysis
Differences were assessed for statistical significance using the Mann-Whitney U test or chi-square test as appropriate (IBM SPSS Version 21).
Sample size
A convenience sample of 40 infants was recruited. Recruitment of 20 infants into each group allowed us to detect a difference of 25 % in the WOB between the groups and differences in the results of the other physiological measurements equivalent to one standard deviation with 80 % power at the 5 % level.
Results
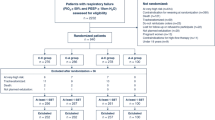
During the study period, there were 102 infants at or near term who were ventilated (Fig. 1). The infants who were not recruited into the study were similar to those who were recruited (Table 1). Forty infants (19 were male) were recruited into the study (Table 2). Approximately half the infants had a surgical condition and were studied post-operatively (Table 3). The median age at randomisation did not differ between the two groups (Table 2). There were no significant differences in the pre-extubation level of respiratory support or pre-extubation PaCO2 levels between the two groups (Table 2). The median time to extubation did not differ between the two groups being 25 (2.5–312) hours in the VTV and 33.5 (1–312) hours in the PLV group, p = 0.1461 (Fig. 2). The one infant who met failure criteria was in the VTV group and consistently required a PIP >30 cmH2O to achieve a tidal volume of 5 ml/kg.
Physiological measurements were not possible in six infants as they were extubated before the measurement could be made. In addition, no measurements were made in seven infants for technical reasons and four infants self-extubated. In some infants, only certain of the measurements could be made; for example, TAA was not attempted in infants with gastroschisis as the bands could not be sited. There were no significant differences in the results of the physiological measurements between the two groups (Table 4).
In the VTV group, a median of 6 (range 2–34) blood gases were obtained, and in the PLV group, a median of 9 (range 3–57) (p = 0.11). In the VT group, there was a median of 1.5 (range 0.8) episodes of hypocarbia compared to a median of 4 (range 1–13) in the PLV group (p = 0.005).
Discussion
We have demonstrated no significant differences in the time to successful extubation in at or near term-born infants supported by VTV or PLV. We recruited a convenience sample which only allowed detection of a difference of 70 h in the time to successful extubation between the two groups, but unexpectedly, the majority of infants were ventilated for less than that time. This perhaps reflects approximately half had surgical conditions and some were extubated very soon after the surgical intervention. In contrast, some “surgical” infants required many days of ventilatory support, as has been our previous experience. We demonstrated no significant differences in the results of physiological measurements performed prior to extubation, but we were able only to perform the measurements in a proportion of the infants. Hence, we cannot exclude that there might have been significant differences between the two groups in the results of the physiological measurements if had we been able to study all 40 patients. In addition, we cannot exclude that physiological measurements earlier in the infants’ respiratory support career might have detected significant differences between the two groups. VTV, however, was associated with significantly fewer episodes of hypocarbia, despite the number of arterial blood gases being similar in the two groups. Indeed, there was a very high rate of hypocarbic episodes in the PLV group. Hypocarbia is associated with PVL in prematurely born infants [19] and PLV had been reported in near term-born infants [12]. In addition, a poorer outcome has been documented in term-born infants with post-asphyxial hypoxic ischaemic encephalopathy exposed to severe hypocarbia [13, 16]. Reduction in episodes of hypocarbia in at or near term-born infants then is an important outcome.
We used a VT level of 5 ml/kg as it has been shown that it is associated with a lower WOB in infants born at or near term than 4 ml/kg [6]. Use of a higher VT (6 ml/kg) resulted in a significantly lower WOB [6], but we were reluctant to use that level as it might have resulted in impaired respiratory muscle strength.
There have been a few studies examining different ventilator rates during PLV, but to our knowledge, only two included term as well as prematurely born infants. In one study [10], ventilation at a rate of 60 bpm with an inspiratory time of 0.5 s was compared to a rate of 20–40 bpm with an inspiratory time of 1 s. The number of term-born infants included in the study was not stated, but the pneumothoraces all occurred in infants of birth weight less than 1.7 kg [10]. In another study (OCTAVE) [15], rates of 60 bpm were compared to rates of 20–40 bpm, the number of term born infants was not stated. No significant difference in the pneumothorax rate was demonstrated overall. Asynchrony can also be avoided by using patient triggered modes, but to our knowledge, there are only two such studies which have included term as well as preterm infants and neither showed significant differences in air leaks. In one [3], there were no significant differences in the duration of ventilation, need for reintubation or pneumothorax or mortality rates between infants supported by synchronised intermittent mandatory ventilation (SIMV) or IMV. Only 15 infants born at term (all with meconium aspiration syndrome) were included [3]. In the other [2], 327 infants were randomised to SIMV or IMV. Ninety-three infants with a birth weight greater than 2 k and a mean gestational age 36 weeks, and the study was adequately powered for subgroup analysis with respect to the oxygenation index and the incidence of air leaks. Amongst that subset, those supported by SIMV had a shorter duration of ventilation (p = 0.02) but had similar rates of death, oxygen dependency at 28 days and air leaks to the SIMV group [2].
Our study has a number of strengths and some limitations. The same ventilator type was used in both arms of the study; performance differs according to type of ventilator delivering VTV with regard to airway pressure waveforms [5]. We recruited consecutive infants who fulfilled the eligibility criteria, and a researcher was available to obtain informed written consent. A criteria for failure of the randomised mode was a peak inflating pressure greater than 30 cmH2O; interestingly, only one infant met that criteria. It has been previously reported in a study of 70,000 infants [9] that the overall incidences per 1000 live births of requirement for mechanical ventilation were 0.72 % for transient tachypnoea of the newborn (TTN), 0.38 % for respiratory distress syndrome (RDS) and 0.61 % for meconium aspiration syndrome (MAS). In our experience, it is rare to see RDS at or near term, but similarly, our study included infants with TTN and MAS. We compared VTV to PLV rather than a triggered mode, as no RCT has demonstrated better outcomes for triggered modes with regard to the duration of ventilation or need for reintubation in at or near term-born infants [2, 3]. Similar numbers of infants in each of the randomised arms received intravenous morphine, thus it seems unlikely this influenced the TAA results. Only 40 % of the 81 eligible infants were included in the study, but comparison of those who were and were not recruited did not reveal any significant differences between the groups. The clinicians were not blinded to the intervention and indeed were not in any of the studies included in the meta-analyses [17, 21]. The lack of significant differences in most of our results would suggest that the lack of blinding had not influenced the performance of the study. A limitation of our study may be perceived to be the heterogeneity of the population studied, yet a strength is that they reflect the diagnoses of the currently ventilated born at or near term infants. In addition, in such a heterogenous term-born population, we report a significant difference in an important outcome, the number of episodes of hypocarbia which influences long-term morbidity in such a population [13, 16]. Approximately half of the infants included in this study had surgical conditions, which reflects that our unit is a tertiary surgical and medical referral centre. We did not, however, undertake a subanalysis according to medical versus surgical diagnosis as our sample size was not calculated to allow such a subanalysis. Our results demonstrate a significant difference in hypocarbic episodes overall.
We recently undertook a survey of respiratory support practices in infants born at term [7] which demonstrated that 26 % of NICUs and 11 % of local neonatal units (LNUs) used VTV routinely in infants born at term. A wide variety of volume target levels (3–10 ml/kg) was used [7]. Yet, there have been no RCTs investigating whether VTV has benefits for at or near term-born infants. The data from our RCT is then useful to inform practitioners regarding the possible benefits of VTV.
In conclusion, in infants born at or near term, VTV compared to PLV as implemented using the SLE ventilator did not improve the time to reach successful extubation or the results of physiological assessments. VTV, however, was associated with significantly fewer hypocarbia episodes, and hence, we would recommend its use for infants born at or near term.
Abbreviations
- HFOV:
-
High-frequency oscillatory ventilation
- IVH:
-
Intraventricular haemorrhage
- Pemax :
-
Maximum expiratory pressure
- Pimax :
-
Maximum inflation pressure
- PIP:
-
Peak inspiratory pressure
- PLV:
-
Pressure-limited ventilation
- PTPdi:
-
Transdiaphragmatic pressure time product
- PVL:
-
Periventricular leukomalacia
- TAA:
-
Thoracoabdominal asynchrony
- VTV:
-
Volume-targeted ventilation
- WOB:
-
Work of breathing
References
Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH (2001) Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Respir Crit Care Med 14:1154–60
Bernstein G, Mannino FL, Heldt GP, Callahan JD, Bull DH, Sola A, Ariagno RL, Hoffman GL, Frantz ID 3rd, Troche BI, Roberts JL, Dela Cruz TV, Costa E (1996) Randomized multicenter trial comparing synchronized and conventional intermittent mandatory ventilation in neonates. J Pediatr 128:453–63
Chen JY, Ling UP, Chen JH (1997) Comparison of synchronized and conventional intermittent mandatory ventilation in neonates. Acta Paediatr Jpn 39:578–83
Chowdhury O, Greenough A (2011) Neonatal ventilatory techniques—which are best for infants born at term? Arch Med Sci 7:381–7
Chowdhury O, Patel DS, Hannam S, Lee S, Rafferty GF, Peacock JL, Greenough A (2013) Randomised trial of volume targeted ventilation versus pressure limited ventilation in acute respiratory failure in prematurely born infants. Neonatol 104:290–4
Chowdhury O, Rafferty GF, Lee S, Hannam S, Milner AD, Greenough A (2012) Volume targeted ventilation in infants born at or near term. Arch Dis Child Fetal Neonatal Ed 97:F264–6
Chowdhury O, Wedderburn CJ, Lee S, Hannam S, Greenough A (2012) Respiratory support practices in infants born at term in the United Kingdom. Eur J Pediatr 171:1633–8
Clark RH (2005) The epidemiology of respiratory failure in neonates born at an estimated gestational age of 34 weeks or more. J Perinatol 25:251–7
Gouyon JB, Ribakovsky C, Ferdynus C, Quantin C, Sagot P, Gouyon B, Network BP (2008) Severe respiratory disorders in term neonates. Paediatr Perinat Epidemiol 22:22–30
Heicher DA, Kasting DS, Harrod JR (1981) Prospective clinical comparison of two methods for mechanical ventilation of neonates: rapid rate and short inspiratory time versus slow rate and long inspiratory time. J Pediatr 98:957–61
Hird M, Greenough A, Gamsu HR (1990) Gas trapping during high frequency positive pressure ventilation using conventional ventilators. Early Hum Dev 22:51–6
Kinney HC (2006) The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol 30:81–8
Klinger G, Beyene J, Shah P, Perlman M (2005) Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia. Arch Dis Child Fetal Neonatal Ed 90:F49–52
Lian AnnLian W, Yeo CL, Ho LY (2002) Two year outcome of normal birth weight infants admitted to a Singapore neonatal intensive care unit. Ann Acad Med Singapore 31:199–205
Oxford Region Controlled Trial of Artificial Ventilation OCTAVE Study Group (1991) Multicentre randomised controlled trial of high against low frequency positive pressure ventilation. Arch Dis Child 66:770–5
Pappas A, Shankaran S, Laptook AR, Langer JC, Bara R, Ehrenkranz RA, Goldberg N, Das A, Higgins RD, Tyson JE, Walsh MC, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (2011) Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J Pediatr 158:752–8
Peng W, Zhu H, Shi H, Liu E (2014) Volume-targeted ventilation is more suitable than pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 99:F158–65
Ramadan G, Paul N, Morton M, Peacock JL, Greenough A (2012) Outcome of ventilated infants born at term without major congenital abnormalities. Eur J Pediatr 171:331–6
Shankaran S, Langer JC, Kazzi SN, Laptook AR, Walsh M, National Institute of Child Health and Human Development Neonatal Research Network: Neonatal Research Network (2006) Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia in low birth weight infants. Pediatrics 118:1654–9
Sutton L (1997) Population based data on full term neonates with severe morbidity. Semin Neonatol 2:189–93
Wheeler K, Klingenberg C, McCallion N, Morley CJ, Davis PG (2010) Volume targeted versus pressure limited ventilation in the neonate. Cochrane Database Syst Rev 11:CD003666
Acknowledgements
Authors’ contributions
AG, SH and GFR designed the study, PB, OC and SS collected the data, JLP contributed statistical expertise. All authors were involved in production of the final manuscript.
Compliance with ethical standards
ᅟ
Funding
Dr Prashanth Bhat and Dr Olie Chowdhury were supported by the Charles Wolfson Charitable Trust. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
AG has held grants from various ventilator manufacturers; AG has received honoraria for giving lectures and advising various ventilator manufacturers. SH has received sponsorship for postgraduate courses from ventilator manufacturers. PB, OC, SS, GFR and JLP have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained for the parents of all infants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Patrick Van Reempts
Revisions received: 11 June 2015 / 07 July 2015
Rights and permissions
About this article
Cite this article
Bhat, P., Chowdhury, O., Shetty, S. et al. Volume-targeted versus pressure-limited ventilation in infants born at or near term. Eur J Pediatr 175, 89–95 (2016). https://doi.org/10.1007/s00431-015-2596-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2596-3